Methionine in proteins: The Cinderella of the proteinogenic amino acids
- PMID: 31359525
- PMCID: PMC6739822
- DOI: 10.1002/pro.3698
Methionine in proteins: The Cinderella of the proteinogenic amino acids
Abstract
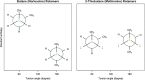
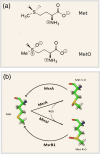
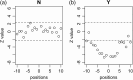
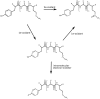
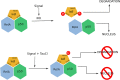
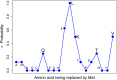
Methionine in proteins, apart from its role in the initiation of translation, is assumed to play a simple structural role in the hydrophobic core, in a similar way to other hydrophobic amino acids such as leucine, isoleucine, and valine. However, research from a number of laboratories supports the concept that methionine serves as an important cellular antioxidant, stabilizes the structure of proteins, participates in the sequence-independent recognition of protein surfaces, and can act as a regulatory switch through reversible oxidation and reduction. Despite all these evidences, the role of methionine in protein structure and function is largely overlooked by most biochemists. Thus, the main aim of the current article is not so much to carry out an exhaustive review of the many and diverse processes in which methionine residues are involved, but to review some illustrative examples that may help the nonspecialized reader to form a richer and more precise insight regarding the role-played by methionine residues in such processes.
Keywords: MetOSite; methionine sulfoxide; posttranslational modification; protein oxidation.
© 2019 The Protein Society.
Figures

References
-
- Gellman SH. On the role of methionine residues in the sequence‐independent recognition of nonpolar protein surfaces. Biochemistry. 1991;30:6633–6636. - PubMed
-
- Janin J, Wodak S. Conformation of amino acid side‐chains in proteins. J Mol Biol. 1978;125:357–386. - PubMed
-
- Alvarez‐Carreño C, Becerra A, Lazcano A. Norvaline and norleucine may have been more abundant protein components during early stages of cell evolution. Orig Life Evol Biosph. 2013;43:363–375. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

