TLX3 repressed SNAI1-induced epithelial-mesenchymal transition by directly constraining STAT3 phosphorylation and functionally sensitized 5-FU chemotherapy in hepatocellular carcinoma
- PMID: 31360112
- PMCID: PMC6643223
- DOI: 10.7150/ijbs.33844
TLX3 repressed SNAI1-induced epithelial-mesenchymal transition by directly constraining STAT3 phosphorylation and functionally sensitized 5-FU chemotherapy in hepatocellular carcinoma
Abstract
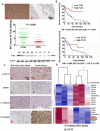
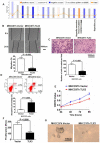
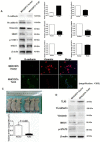
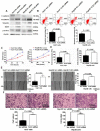
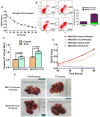
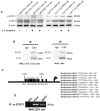
TLX3 has an established role as a sequence-specific transcription factor with vital functions in the nervous system. Although several studies have shown that TLX3 is aberrantly up-regulated in leukemia, its expression and function in hepatocellular carcinoma (HCC) remain unknown. We found that TLX3 expression was decreased in 68/100 (68%) HCC cases and negatively correlated with the expression of p-STAT3, SNAI1, and Vimentin, while it was positively associated with E-cadherin expression. ITRAQ proteomic profiling revealed significantly less TLX3 expression in primary HCC tumors than in portal vein tumor thrombi. Comparison of Kaplan-Meier curves showed that down-regulation of TLX3 in HCC was associated with poor post-surgical survival. TLX3 over-expression inhibited HCC cell viability, proliferation, migration, invasion and enhanced 5-FU treatment, whereas silencing TLX3 produced the opposite results. Further experiments showed that TLX3 attenuated the EMT phenotype. In vivo experiments showed that knockdown of TLX3 promoted the growth of HCC xenografts and attenuated the anti-tumor effects of 5-FU treatment. Gene expression microarray analysis revealed that TLX3 inhibited IL-6/STAT3 signaling. In additional mechanistic studies TLX3 reversed the EMT phenotype of HCC cells by binding to STAT3, inhibiting STAT3 phosphorylation, and down-regulating SNAI1 expression. Taken together, loss of expression of TLX3 induces EMT by enhancing IL-6/STAT3/SNAI1 signaling, and accelerates HCC progression while also attenuated the effect of 5-FU on HCCs.
Keywords: EMT; HCC; SNAI1; STAT3; TLX3.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
Deciphering STAT3 signaling potential in hepatocellular carcinoma: tumorigenesis, treatment resistance, and pharmacological significance.Cell Mol Biol Lett. 2023 Apr 21;28(1):33. doi: 10.1186/s11658-023-00438-9. Cell Mol Biol Lett. 2023. PMID: 37085753 Free PMC article. Review.
-
FoxM1 overexpression promotes epithelial-mesenchymal transition and metastasis of hepatocellular carcinoma.World J Gastroenterol. 2015 Jan 7;21(1):196-213. doi: 10.3748/wjg.v21.i1.196. World J Gastroenterol. 2015. PMID: 25574092 Free PMC article.
-
RBFOX3 Regulates the Chemosensitivity of Cancer Cells to 5-Fluorouracil via the PI3K/AKT, EMT and Cytochrome-C/Caspase Pathways.Cell Physiol Biochem. 2018;46(4):1365-1380. doi: 10.1159/000489153. Epub 2018 Apr 18. Cell Physiol Biochem. 2018. PMID: 29689552
-
Coexpression of gene Oct4 and Nanog initiates stem cell characteristics in hepatocellular carcinoma and promotes epithelial-mesenchymal transition through activation of Stat3/Snail signaling.J Hematol Oncol. 2015 Mar 11;8:23. doi: 10.1186/s13045-015-0119-3. J Hematol Oncol. 2015. PMID: 25879771 Free PMC article.
-
Interleukin-6 at the Host-Tumor Interface: STAT3 in Biomolecular Condensates in Cancer Cells.Cells. 2022 Mar 30;11(7):1164. doi: 10.3390/cells11071164. Cells. 2022. PMID: 35406728 Free PMC article. Review.
Cited by
-
Deciphering STAT3 signaling potential in hepatocellular carcinoma: tumorigenesis, treatment resistance, and pharmacological significance.Cell Mol Biol Lett. 2023 Apr 21;28(1):33. doi: 10.1186/s11658-023-00438-9. Cell Mol Biol Lett. 2023. PMID: 37085753 Free PMC article. Review.
-
Mettl3 Mediated m6A Methylation Involved in Epithelial-Mesenchymal Transition by Targeting SOCS3/STAT3/SNAI1 in Cigarette Smoking-Induced COPD.Int J Chron Obstruct Pulmon Dis. 2023 May 30;18:1007-1017. doi: 10.2147/COPD.S398289. eCollection 2023. Int J Chron Obstruct Pulmon Dis. 2023. PMID: 37275442 Free PMC article.
-
Sulfatase 2-Induced Cancer-Associated Fibroblasts Promote Hepatocellular Carcinoma Progression via Inhibition of Apoptosis and Induction of Epithelial-to-Mesenchymal Transition.Front Cell Dev Biol. 2021 Apr 6;9:631931. doi: 10.3389/fcell.2021.631931. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33889573 Free PMC article.
-
BP1003 Decreases STAT3 Expression and Its Pro-Tumorigenic Functions in Solid Tumors and the Tumor Microenvironment.Biomedicines. 2024 Aug 20;12(8):1901. doi: 10.3390/biomedicines12081901. Biomedicines. 2024. PMID: 39200368 Free PMC article.
-
N6-methyladenosine reader YTHDF3-mediated zinc finger protein 41 inhibits hepatocellular carcinoma progression by transcriptional repression of Snail.MedComm (2020). 2024 Oct 28;5(11):e763. doi: 10.1002/mco2.763. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39473907 Free PMC article.
References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Song P, Tang W, Hasegawa K, Kokudo N. Systematic evidence-based clinical practice guidelines are ushering in a new stage of standardized management of hepatocellular carcinoma in Japan. Drug Discov Ther. 2014;8:64–70. - PubMed
-
- Roayaie S, Jibara G, Tabrizian P, Park JW, Yang J, Yan L. et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology. 2015;62:440–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

