Serum microRNA Signature Is Capable of Early Diagnosis for Non-Small Cell Lung Cancer
- PMID: 31360113
- PMCID: PMC6643220
- DOI: 10.7150/ijbs.33986
Serum microRNA Signature Is Capable of Early Diagnosis for Non-Small Cell Lung Cancer
Abstract
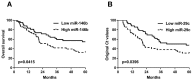
Despite decades of efforts, non-small-cell lung cancer (NSCLC) remains the leading cause of cancer mortality globally primarily due to the challenge in early detection of the cancer. Being an important player in cancer development, the dysregulated miRNAs have been shown promising values as non-invasive diagnostic and prognostic biomarkers for NSCLC. The aim of our study is to access the efficacy and reliability of a potential circulating miRNA panel in early diagnosis of NSCLC. We first selected eight candidate miRNAs, miR-146b, miR-205, miR-29c, miR-31, miR-30b, miR-337, miR-411, and miR-708, which have been shown frequently aberrant in primary NSCLC patients based on our previous studies and other reports. The serum level of each of these miRNAs was evaluated by quantitative real-time PCR (qRT-PCR) in training and testing sets. We found that 5 out of 8 miRNAs (miR-146b, miR-205, miR-29c, miR-30b, and miR-337) were significantly up-regulated in NSCLCs patients compared to healthy or cancer-free controls in both training and testing sets. Based on the logistic regression model, a 4-miRNAs set (miR-146b, miR-205, miR-29c and miR-30b) was picked out of the 5 miRNAs owing to its excellent diagnostic power for NSCLC patients in the training set (AUC=0.99, accuracy=95.00%), the testing set (AUC=0.93, accuracy=89.69%), and the training-testing combined set ( AUC=0.96, accuracy=92.00%). When pathological subtypes of NSCLC are compared, this 4-miRNA panel carried a relatively higher prediction power and higher sensitivity for adenocarcinoma (AC) (AUC=0.98, sensitivity=99.10%) than for squamous cell carcinoma (SCC) (AUC=0.93, sensitivity=90.32%). Additionally, this panel demonstrated a comparable diagnostic capacity for stage I (AUC=0.96) and stage II-III (AUC=0.95) of NSCLC, suggesting its role in reflecting the tumor load. Importantly, the high levels of miR-146b and miR-29c in serum were significantly associated with poor 5-year overall survival (OS) (both p=0.04). Further survival analysis showed that high level of miR-146b in serum is specifically correlated with poor survival rate in SCC patients (p=0.0035) but not in AC patients (p=0.83), consistent with our previous finding that the high tissue expression of miR-146b in lung cancer specimen is indicative of a poor prognosis for SCC patients. Altogether, our study demonstrated that the 4-miRNA panel is a novel, sensitive and non-invasive serum marker for the early diagnosis of NSCLC.
Keywords: early diagnosis; miRNA; non-small-cell lung cancer (NSCLC); prognosis; serum biomarker.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
Serum miR-223: A Validated Biomarker for Detection of Early-Stage Non-Small Cell Lung Cancer.Cancer Epidemiol Biomarkers Prev. 2019 Nov;28(11):1926-1933. doi: 10.1158/1055-9965.EPI-19-0626. Epub 2019 Sep 5. Cancer Epidemiol Biomarkers Prev. 2019. PMID: 31488416
-
Potential circulating miRNA signature for early detection of NSCLC.Cancer Genet. 2017 Oct;216-217:150-158. doi: 10.1016/j.cancergen.2017.07.006. Epub 2017 Aug 7. Cancer Genet. 2017. PMID: 29025589
-
A novel circulating miRNA-based signature for the early diagnosis and prognosis prediction of non-small-cell lung cancer.J Clin Lab Anal. 2020 Nov;34(11):e23505. doi: 10.1002/jcla.23505. Epub 2020 Aug 9. J Clin Lab Anal. 2020. PMID: 33463758 Free PMC article.
-
Identification of differentially expressed circulating serum microRNA for the diagnosis and prognosis of Indian non-small cell lung cancer patients.Curr Probl Cancer. 2020 Aug;44(4):100540. doi: 10.1016/j.currproblcancer.2020.100540. Epub 2020 Jan 23. Curr Probl Cancer. 2020. PMID: 32007320 Review.
-
Clinical use of microRNAs as potential non-invasive biomarkers for detecting non-small cell lung cancer: a meta-analysis.Respirology. 2015 Jan;20(1):56-65. doi: 10.1111/resp.12444. Epub 2014 Dec 1. Respirology. 2015. PMID: 25440223 Review.
Cited by
-
Recent Advances in Lung Cancer Therapy Based on Nanomaterials: A Review.Curr Med Chem. 2023;30(3):335-355. doi: 10.2174/0929867328666210810160901. Curr Med Chem. 2023. PMID: 34375182 Review.
-
Testing the accuracy of a three-miRNA panel for the detection of primary prostate cancer: a discovery and validation study.Future Oncol. 2025 Jul;21(17):2167-2176. doi: 10.1080/14796694.2025.2514930. Epub 2025 Jun 6. Future Oncol. 2025. PMID: 40474836 Free PMC article.
-
The Double Face of miR-708: A Pan-Cancer Player with Dissociative Identity Disorder.Genes (Basel). 2022 Dec 16;13(12):2375. doi: 10.3390/genes13122375. Genes (Basel). 2022. PMID: 36553642 Free PMC article. Review.
-
Extracellular vesicles in triple-negative breast cancer: current updates, challenges and future prospects.Front Mol Biosci. 2025 Apr 14;12:1561464. doi: 10.3389/fmolb.2025.1561464. eCollection 2025. Front Mol Biosci. 2025. PMID: 40297849 Free PMC article. Review.
-
Simulated Microgravity Effects on Human Adenocarcinoma Alveolar Epithelial Cells: Characterization of Morphological, Functional, and Epigenetic Parameters.Int J Mol Sci. 2021 Jun 28;22(13):6951. doi: 10.3390/ijms22136951. Int J Mol Sci. 2021. PMID: 34203322 Free PMC article.
References
-
- Bray F, Ferlay J, Soerjomataram I. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. - PubMed
-
- Wood SL, Pernemalm M, Crosbie PA. et al. Molecular histology of lung cancer: from targets to treatments. Cancer Treat Rev. 2015;41:361–375. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

