Role of epigenetic modifications in the aberrant CYP19A1 gene expression in polycystic ovary syndrome
- PMID: 31360184
- PMCID: PMC6657255
- DOI: 10.5114/aoms.2019.86060
Role of epigenetic modifications in the aberrant CYP19A1 gene expression in polycystic ovary syndrome
Abstract
Introduction: In this study, the global DNA methylation, histone acetylation and methylation levels of cumulus cells (CCs) in infertile polycystic ovary syndrome (PCOS) patients and the correlation of these epigenetic modifications with the expression of the ovarian aromatase gene (as an important marker in the etiology of PCOS) were investigated.
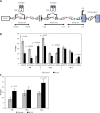
Material and methods: A cross-sectional study was conducted on 24 patients (12 PCOS patients and 12 healthy women), who underwent ovarian stimulation. Nucleosome ELISA was performed, in order to identify the global occupancy level of Mecp2 (as a marker of DNA methylation) and H3K9me2/H3K9ac as histone modification markers in chromatin fractions obtained from CCs. The CYP19A1 gene expression was measured by qRT-PCR. The level of DNA incorporation of MeCP2, histone modification markers and binding of estrogen receptor β (ERβ) to CYP19A1 regulatory sequences were examined by ChIP-QPCR assay.
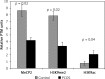
Results: The data demonstrate a significant increase in global occupancy levels of MeCP2 and H3K9ac markers and a decrease of H3K9me2 to chromatin in CCs of PCOS patients vs. control group. Furthermore, CYP19A1 gene expression, and the incorporation of H3K9ac in PII, PI.3, and PI.4 promoters of CYP19A1 in PCOS, were higher than those of controls. Also, significant hypomethylation of H3K9 at PII and DNA hypomethylated at PII and PI.3 promoters and differential binding of ERβ to three promoters were observed in PCOS patients (p < 0.05).
Conclusions: Aromatase expression can be affected by epigenetic modifications and differential ERβ binding to the proximal CYP19A1 promoters. These mechanisms may be involved in the enhanced aromatase transcription during ovarian stimulation in PCOS patients.
Keywords: CYP19A1; cumulus cell; epigenetic; estrogen receptor β; polycystic ovary syndrome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Epigenetic alterations of CYP19A1 gene in Cumulus cells and its relevance to infertility in endometriosis.J Assist Reprod Genet. 2016 Aug;33(8):1105-13. doi: 10.1007/s10815-016-0727-z. Epub 2016 May 11. J Assist Reprod Genet. 2016. PMID: 27167072 Free PMC article.
-
Promoter methylation of CYP19A1 gene in Chinese polycystic ovary syndrome patients.Gynecol Obstet Invest. 2013;76(4):209-13. doi: 10.1159/000355314. Epub 2013 Oct 19. Gynecol Obstet Invest. 2013. PMID: 24157654
-
Deregulation of RUNX2 by miR-320a deficiency impairs steroidogenesis in cumulus granulosa cells from polycystic ovary syndrome (PCOS) patients.Biochem Biophys Res Commun. 2017 Jan 22;482(4):1469-1476. doi: 10.1016/j.bbrc.2016.12.059. Epub 2016 Dec 11. Biochem Biophys Res Commun. 2017. PMID: 27965096
-
CYP19A1 Promoters Activity in Human Granulosa Cells: A Comparison between PCOS and Normal Subjects.Cell J. 2022 Apr;24(4):170-175. doi: 10.22074/cellj.2022.7787. Epub 2022 Apr 27. Cell J. 2022. PMID: 35674020 Free PMC article.
-
DNA methylation in the pathogenesis of polycystic ovary syndrome.Reproduction. 2019 Jul;158(1):R27-R40. doi: 10.1530/REP-18-0449. Reproduction. 2019. PMID: 30959484 Review.
Cited by
-
Association of CYP19A1 Gene, Plasma Zinc, and Urinary Zinc with the Risk of Type 2 Diabetes Mellitus in a Chinese Population.Biol Trace Elem Res. 2023 Sep;201(9):4205-4215. doi: 10.1007/s12011-022-03502-1. Epub 2022 Nov 28. Biol Trace Elem Res. 2023. PMID: 36441497
-
Estrogen-Receptor Expression and Function in Female Reproductive Disease.Cells. 2019 Sep 21;8(10):1123. doi: 10.3390/cells8101123. Cells. 2019. PMID: 31546660 Free PMC article. Review.
-
Role of Epigenetic Modification in the Intergeneration Transmission of War Trauma.Indian J Clin Biochem. 2024 Jul;39(3):312-321. doi: 10.1007/s12291-023-01136-1. Epub 2023 May 12. Indian J Clin Biochem. 2024. PMID: 39005862 Free PMC article. Review.
-
Polycystic Ovary Syndrome: the Epigenetics Behind the Disease.Reprod Sci. 2022 Mar;29(3):680-694. doi: 10.1007/s43032-021-00516-3. Epub 2021 Apr 7. Reprod Sci. 2022. PMID: 33826098 Review.
-
The effect of clomiphene citrate, herbal mixture, and herbal mixture along with clomiphene citrate on clinical and para-clinical parameters in infertile women with polycystic ovary syndrome: a randomized controlled clinical trial.Arch Med Sci. 2020 Feb 25;16(6):1304-1318. doi: 10.5114/aoms.2020.93271. eCollection 2020. Arch Med Sci. 2020. PMID: 33224329 Free PMC article.
References
-
- McVeigh E, Guillebaud J, Homburg R. Oxford Handbook of Reproductive Medicine and Family Planning. 2nd ed. Oxford: Oxford University Press;
-
- Diamanti-Kandarakis E. Polycystic ovarian syndrome: pathophysiology, molecular aspects and clinical implications. Expert Rev Mol Med. 2008;10:e3. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
