Fluorescent probes for organelle-targeted bioactive species imaging
- PMID: 31360411
- PMCID: PMC6585876
- DOI: 10.1039/c9sc01652j
Fluorescent probes for organelle-targeted bioactive species imaging
Abstract
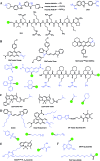
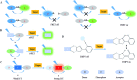
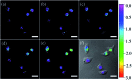
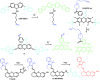
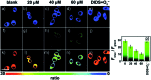
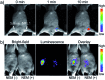
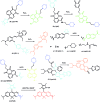
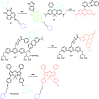
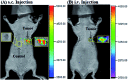
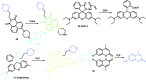
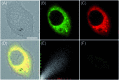
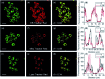
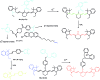
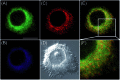
Bioactive species, including reactive oxygen species (ROS, including O2˙-, H2O2, HOCl, 1O2, ˙OH, HOBr, etc.), reactive nitrogen species (RNS, including ONOO-, NO, NO2, HNO, etc.), reactive sulfur species (RSS, including GSH, Hcy, Cys, H2S, H2S n , SO2 derivatives, etc.), ATP, HCHO, CO and so on, are a highly important category of molecules in living cells. The dynamic fluctuations of these molecules in subcellular microenvironments determine cellular homeostasis, signal conduction, immunity and metabolism. However, their abnormal expressions can cause disorders which are associated with diverse major diseases. Monitoring bioactive molecules in subcellular structures is therefore critical for bioanalysis and related drug discovery. With the emergence of organelle-targeted fluorescent probes, significant progress has been made in subcellular imaging. Among the developed subcellular localization fluorescent tools, ROS, RNS and RSS (RONSS) probes are highly attractive, owing to their potential for revealing the physiological and pathological functions of these highly reactive, interactive and interconvertible molecules during diverse biological events, which are rather significant for advancing our understanding of different life phenomena and exploring new technologies for life regulation. This review mainly illustrates the design principles, detection mechanisms, current challenges, and potential future directions of organelle-targeted fluorescent probes toward RONSS.
Figures
















References
-
- Carter K. P., Young A. M., Palmer A. E. Chem. Rev. 2014;114:4564–4601. - PMC - PubMed
- Kim H. M., Cho B. R. Chem. Rev. 2015;115:5014–5055. - PubMed
- Liu J., Cao Z., Lu Y. Chem. Rev. 2009;109:1948–1998. - PMC - PubMed
- Guo Z., Park S., Yoon J., Shin I. Chem. Soc. Rev. 2014;43:16–29. - PubMed
- Lin V. S., Chen W., Xian M., Chang C. J. Chem. Soc. Rev. 2015;44:4596–4618. - PMC - PubMed
- Niu L.-Y., Chen Y.-Z., Zheng H.-R., Wu L.-Z., Tung C.-H., Yang Q.-Z. Chem. Soc. Rev. 2015;44:6143–6160. - PubMed
- Wu D., Sedgwick A. C., Gunnlaugsson T., Akkaya E. U., Yoon J., James T. D. Chem. Soc. Rev. 2017;46:7105–7123. - PubMed
- Heffern M. C., Matosziuk L. M., Meade T. J. Chem. Rev. 2013;114:4496–4539. - PMC - PubMed
-
- Chan J., Dodani S. C., Chang C. J. Nat. Chem. 2012;4:973–984. - PMC - PubMed
- Chyan W., Zhang D. Y., Lippard S. J., Radford R. J. Proc. Natl. Acad. Sci. U. S. A. 2014;111:143–148. - PMC - PubMed
- Liu H. W., Chen L., Xu C., Li Z., Zhang H., Zhang X. B., Tan W. Chem. Soc. Rev. 2018;47:7140–7180. - PubMed
- Qian X., Xu Z. Chem. Soc. Rev. 2015;44:4487–4493. - PubMed
- Sedgwick A. C., Wu L., Han H. H., Bull S. D., He X. P., James T. D., Sessler J. L., Tang B. Z., Tian H., Yoon J. Chem. Soc. Rev. 2018;47:8842–8880. - PubMed
- Tang Y., Lee D., Wang J., Li G., Yu J., Lin W., Yoon J. Chem. Soc. Rev. 2015;44:5003–5015. - PubMed
- Roopa, Kumar N., Bhalla V., Kumar M. Chem. Commun. 2015;51:15614–15628. - PubMed
-
- Xu W., Zeng Z., Jiang J.-H., Chang Y.-T., Yuan L. Angew. Chem., Int. Ed. 2016;55:13658–13699. - PubMed
- Zhu H., Fan J., Du J., Peng X. Acc. Chem. Res. 2016;49:2115–2126. - PubMed
- Jiao X., Li Y., Niu J., Xie X., Wang X., Tang B. Anal. Chem. 2018;90:533–555. - PubMed
- Xu Z., Xu L. Chem. Commun. 2016;52:1094–1119. - PubMed
- Zhang R., Song B., Yuan J. TrAC, Trends Anal. Chem. 2018;99:1–33.
-
- Zielonka J., Joseph J., Sikora A., Hardy M., Ouari O., Vasquez-Vivar J., Cheng G., Lopez M., Kalyanaraman B. Chem. Rev. 2017;117:10043–10120. - PMC - PubMed
- Fernandez A., Vendrell M. Chem. Soc. Rev. 2016;45:1182–1196. - PubMed
- Lee M. H., Sharma A., Chang M. J., Lee J., Son S., Sessler J. L., Kang C., Kim J. S. Chem. Soc. Rev. 2018;47:28–52. - PMC - PubMed
- Qiu K., Chen Y., Rees T. W., Ji L., Chao H. Coord. Chem. Rev. 2019;378:66–86.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

