Advanced Lipid Technologies® (ALT®): A Proven Formulation Platform to Enhance the Bioavailability of Lipophilic Compounds
- PMID: 31360551
- PMCID: PMC6644232
- DOI: 10.1155/2019/1957360
Advanced Lipid Technologies® (ALT®): A Proven Formulation Platform to Enhance the Bioavailability of Lipophilic Compounds
Abstract
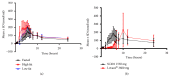
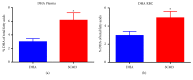
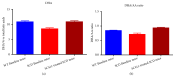
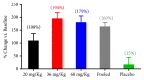
Despite recent advances, the drug development process continues to face significant challenges to efficiently improve the poor solubility of active pharmaceutical ingredients (API) in aqueous media or to improve the bioavailability of lipid-based formulations. The inherent high intra- and interindividual variability of absorption of oral lipophilic drug leads to inconsistent and unpredictable bioavailability and magnitude of the therapeutic effect. For this reason, the development of lipid-based drugs remains a challenging endeavour with a high risk of failure. Therefore, effective strategies to assure a predictable, consistent, and reproducible bioavailability and therapeutic effect for lipid-based medications are needed. Different solutions to address this problem have been broadly studied, including the approaches of particle size reduction, prodrugs, salt forms, cocrystals, solid amorphous forms, cyclodextrin clathrates, and lipid-based drug delivery systems such as self-emulsifying systems and liposomes. Here, we provide a brief description of the current strategies commonly employed to increase the bioavailability of lipophilic drugs and present Advanced Lipid Technologies® (ALT®), a combination of different surfactants that has been demonstrated to improve the absorption of omega-3 fatty acids under various physiological and pathological states.
Figures



References
-
- Delmonte P., Kia A.-R. F., Qing H., Rader J. I. Review of methods for preparation and gas chromatographic separation of trans and cis reference fatty acids. Journal of AOAC International. 2009;92(5):1310–1326. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

