Exclusion of Male Patients in Breast Cancer Clinical Trials
- PMID: 31360850
- PMCID: PMC6649834
- DOI: 10.1093/jncics/pky018
Exclusion of Male Patients in Breast Cancer Clinical Trials
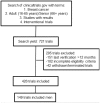
Figures
References
-
- Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2018. CA Cancer J Clin. 2018;681:7–30. - PubMed
-
- Strauss LC, O'Shaughnessy J, Jackson J et al. , . Three parallel randomized phase II trials of dasatinib plus hormone therapy (HT) in advanced ER+ breast cancer (ER+ ABC). J Clin Oncol. 2010;28(15_suppl):TPS133–TPS133.
-
- Reinisch M, Seiler S, Hauzenberger T et al. , . Abstract PD7-10: Male-GBG54: A prospective, randomised multi-centre phase II study evaluating endocrine treatment with either tamoxifen +/- gonadotropin releasing hormone analogue (GnRHa) or an aromatase inhibitor + GnRHa in male breast cancer patients. Cancer Res. 2018;78(4 Supplement):PD7-10-PD7-10.
-
- Giordano SH, Hortobagyi GN.. Leuprolide acetate plus aromatase inhibition for male breast cancer. J Clin Oncol. 2006;2421:e42–e43. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources