Deescalating Adjuvant Trastuzumab in HER2-Positive Early-Stage Breast Cancer: A Systemic Review and Meta-Analysis
- PMID: 31360906
- PMCID: PMC6649709
- DOI: 10.1093/jncics/pkz033
Deescalating Adjuvant Trastuzumab in HER2-Positive Early-Stage Breast Cancer: A Systemic Review and Meta-Analysis
Abstract
Background: One year of adjuvant trastuzumab in combination with chemotherapy is the standard of care in early-stage human epidermal growth factor receptor 2 (HER2)-positive breast cancer. Existing data on shortening trastuzumab treatment show conflicting results.
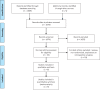
Methods: A search of PubMed and abstracts from key conferences identified randomized trials that compared abbreviated trastuzumab therapy to 1 year of treatment in early-stage HER2-positive breast cancer. Hazard ratios (HRs) and 95% confidence intervals (CIs) were extracted for disease-free survival (DFS) and overall survival (OS). Subgroup analyses evaluated the effect of nodal involvement, estrogen receptor expression, and the duration of abbreviated trastuzumab (9-12 weeks vs 6 months). Odds ratios (ORs) and 95% confidence intervals were computed for prespecified cardiotoxicity events including cardiac dysfunction and congestive heart failure. P values were two-sided.
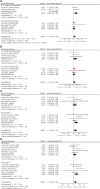
Results: Analysis included six trials comprising 11 603 patients. Shorter trastuzumab treatment was associated with worse DFS (HR = 1.14, 95% CI = 1.05 to 1.25, P = .002) and OS (HR = 1.15, 95% CI = 1.02 to 1.29. P = .02). The effect on DFS was not influenced by estrogen receptor status (P for the subgroup difference = .23), nodal involvement (P = .44), or the different duration of trastuzumab in the experimental arm (P = .09). Shorter trastuzumab treatment was associated with lower odds of cardiac dysfunction (OR = 0.67, 95% CI = 0.55 to 0.81, P < .001) and congestive heart failure (OR = 0.66, 95% CI = 0.50 to 0.86, P = .003).
Conclusions: Compared with 1 year, shorter duration of adjuvant trastuzumab is associated with statistically significantly worse DFS and OS despite favorable cardiotoxicity profile. One year of targeted HER2 treatment should remain the standard adjuvant treatment in early-stage HER2-positive disease with appropriate cardiac monitoring.
Figures

References
-
- Loibl S, Gianni L.. HER2-positive breast cancer. Lancet. 2017;389(10087):2415–2429. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
