On-Surface Reactive Planarization of Pt(II) Complexes
- PMID: 31361071
- PMCID: PMC6856856
- DOI: 10.1002/anie.201906247
On-Surface Reactive Planarization of Pt(II) Complexes
Abstract
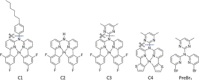
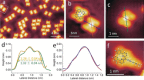
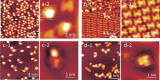
A series of Pt(II) complexes with tetradentate luminophores has been designed, synthesized, and deposited on coinage metal surfaces with the aim to produce highly planar self-assembled monolayers. Low-temperature scanning tunneling microscopy (STM) and density functional theory (DFT) calculations reveal a significant initial nonplanarity for all complexes. A subsequent metal-catalyzed separation of the nonplanar moiety at the bridging unit via the scission of a C-N bond is observed, leaving behind a largely planar core complex. The activation barrier of this bond scission process is found to depend strongly on the chemical nature of both bridging group and coordination plane, and to increase from Cu(111) through Ag(111) to Au(111).
Keywords: Pt complexes; density functional theory calculations; scanning tunnelling microscopy; surface chemistry.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Lu W., Mi B.-X., Chan M. C. W., Hui Z., Che C.-M., Zhu N., Lee S.-T., J. Am. Chem. Soc. 2004, 126, 4958–4971. - PubMed
-
- Fleetham T., Ecton J., Wang Z., Bakken N., Li J., Adv. Mater. 2013, 25, 2573–2576. - PubMed
-
- Baldo M. A., O'Brien D. F., You Y., Shoustikov A., Sibley S., Thompson M. E., Forrest S. R., Nature 1998, 395, 151.
-
- Kim D., Brédas J.-L., J. Am. Chem. Soc. 2009, 131, 11371–11380. - PubMed
-
- Mydlak M., Mauro M., Polo F., Felicetti M., Leonhardt J., Diener G., De Cola L., Strassert C. A., Chem. Mater. 2011, 23, 3659.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

