Recent advances with Treg depleting fusion protein toxins for cancer immunotherapy
- PMID: 31361167
- PMCID: PMC7006781
- DOI: 10.2217/imt-2019-0060
Recent advances with Treg depleting fusion protein toxins for cancer immunotherapy
Abstract
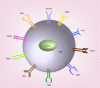
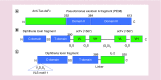
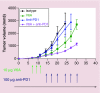
T regulatory cells (Tregs) are an important T cell population for immune tolerance, prevention of autoimmune diseases and inhibition of antitumor immunity. The tumor-promoting role played by Tregs in cancer has prompted numerous approaches to develop immunotherapeutics targeting Tregs. One approach to depletion of Treg cells is retargeting the highly potent cytotoxic activity of bacterial toxins. These agents capitalize on the well-characterized bacterial toxins, diphtheria toxin and Pseudomonas aeruginosa exotoxin A-both of which harbor membrane translocation domains and enzymatic domains that catalytically halt protein synthesis within intoxicated eukaryotic cells and act at picomolar or subpicomolar concentrations. In this review, we summarize the preclinical and clinical development of several Treg-depleting cancer immunotherapies based on these two bacterial toxins.
Keywords: LMB-2; Tregs; anticancer therapy; denileukin diftitox; fusion protein toxins; immunotherapy; melanoma; targeted toxins.
Conflict of interest statement
JR Murphy and W Bishai are cofounders of Sonoval, LLC which holds rights to the commercialization of s-DABIL-2(V6A). The authors acknowledge financial support of a NIH grant AI 130595 and grants from Maryland Tedco, the Cigarette Restitution Fund, and the Abell Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No funded writing assistance was utilized in the production of this manuscript.
Figures


References
-
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 133(5), 775–787 (2008). - PubMed
-
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 10(7), 490–500 (2010). - PubMed
-
- Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 11(1), 7–13 (2010). - PubMed
-
- Shitara K, Nishikawa H. Regulatory T cells: a potential target in cancer immunotherapy. Ann. NY Acad. Sci. 1417(1), 104–115 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
