Adjuvant effect of enterotoxigenic Escherichia coli (ETEC) double-mutant heat-labile toxin (dmLT) on systemic immunogenicity induced by the CFA/I/II/IV MEFA ETEC vaccine: Dose-related enhancement of antibody responses to seven ETEC adhesins (CFA/I, CS1-CS6)
- PMID: 31361177
- PMCID: PMC7062417
- DOI: 10.1080/21645515.2019.1649555
Adjuvant effect of enterotoxigenic Escherichia coli (ETEC) double-mutant heat-labile toxin (dmLT) on systemic immunogenicity induced by the CFA/I/II/IV MEFA ETEC vaccine: Dose-related enhancement of antibody responses to seven ETEC adhesins (CFA/I, CS1-CS6)
Abstract
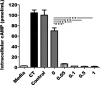
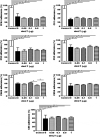
Double-mutant heat-labile toxin (dmLT, LTR192G/L211A) of enterotoxigenic Escherichia coli (ETEC) is an effective mucosal adjuvant. Recent studies have shown that dmLT also exhibits adjuvanticity for antigens administered parenterally. In this study, we subcutaneously (SC) immunized mice with the ETEC adhesin-based vaccine, CFA/I/II/IV MEFA (multiepitope fusion antigen), adjuvanted with dmLT and examined the impact of dmLT on antibody responses specific to the seven adhesins in the vaccine construction [CFA/I, CFA/II (CS1, CS2, CS3) and CFA/IV (CS4, CS5, CS6)]. Mice were immunized with a fixed dose of CFA/I/II/IV MEFA and ascending doses of dmLT adjuvant (0, 0.05, 0.1, 0.5 or 1.0 µg) to assess the potential dmLT dose response relationship. Data showed that dmLT enhanced systemic antibody responses to all seven antigens (CFA/I, CS1-CS6) targeted by MEFA in a dose-dependent way. The adjuvant effect of dmLT on the MEFA construct plateaued at a dose of 0.1 µg. Results also indicated that dmLT is an effective parenteral adjuvant when given by the SC route with the ETEC adhesin MEFA vaccine and that antibody enhancement was achieved with relatively low doses. These observations suggest the potential usefulness of dmLT for parenteral ETEC vaccine candidates and also perhaps for vaccines against other pathogens.
Keywords: CFA/I/II/IV MEFA; Dmlt; adjuvant; antibody response; dose effect; enterotoxigenic Escherichia coli (ETEC).
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
