Neoadjuvant exemestane or exemestane plus docetaxel and cyclophosphamide tailored by clinicopathological response to 12 weeks' exemestane exposure in patients with estrogen receptor-positive breast cancer: A multicenter, open-label, phase II study
- PMID: 31361400
- PMCID: PMC6745863
- DOI: 10.1002/cam4.2423
Neoadjuvant exemestane or exemestane plus docetaxel and cyclophosphamide tailored by clinicopathological response to 12 weeks' exemestane exposure in patients with estrogen receptor-positive breast cancer: A multicenter, open-label, phase II study
Abstract
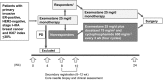
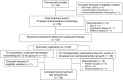
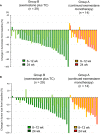
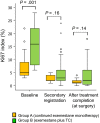
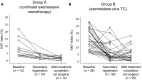
Our aim was to investigate the efficacy and safety of initial neoadjuvant endocrine therapy with exemestane alone followed by tailored treatment, either continued exemestane monotherapy or exemestane plus docetaxel-cyclophosphamide (TC) combination therapy, in postmenopausal patients with primary invasive estrogen receptor-positive, human epidermal growth factor receptor 2-negative, stage I-IIIA breast cancer and Ki67 labeling index ≤30%. In this open-label phase II study, patients initially received exemestane 25 mg/d for 12 weeks. Responders were defined as patients who achieved complete response (CR), partial response (PR) with Ki67 labeling index ≤5% after treatment, or stable disease with Ki67 labeling index ≤5% both before and after treatment. For the subsequent 12 weeks, exemestane monotherapy was continued for responders (group A), whereas nonresponders received exemestane plus four cycles of TC (docetaxel 75 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks) (group B). Clinical response rate (ie the proportion of patients with CR or PR) at 24 weeks was the primary endpoint. Of 64 patients provisionally enrolled between December 2010 and May 2016, 58 (median age 60 years) started the study treatment. Five patients discontinued treatment in the initial exemestane monotherapy period, and 39 completed the study treatment. Clinical response rates at 8-12 and 24 weeks were 71% (10/14, 95% confidence interval [CI] 41.9%-91.6%) and 57% (8/14, 95% CI 28.9%-82.3%), respectively, in group A, and 16% (4/25, 95% CI 4.5%-36.1%) and 56% (14/25, 95% CI 34.9%-75.6%), respectively, in group B. Grade ≥3 adverse events were reported in 8% (1/15) and 53% (20/38) in group A and group B, respectively. The tailored treatment maintained the favorable clinical response to exemestane alone in responders and improved clinical response in nonresponders. TRIAL NUMBER: UMIN000004752 (UMIN Clinical Trials Registry).
Keywords: Ki67 labeling index; aromatase inhibitors; breast neoplasms; docetaxel and cyclophosphamide; tailored therapy.
© 2019 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
NS has received remuneration from Chugai Pharmaceutical, Eisai, Pfizer, and Sysmex. NM has received remuneration from Chugai Pharmaceutical, AstraZeneca, Pfizer, Eli Lilly, Eisai and Takeda Pharmaceutical, and research funding from Chugai, AstraZeneca, Kyowa Hakko Kirin, MSD, Novartis Pharma, Pfizer, Eli Lilly, Eisai and Daiichi Sankyo. TU has received remuneration from Chugai Pharmaceutical, Eisai, and Novartis Pharma. SS has received remuneration from Chugai Pharmaceutical, Novartis Pharma, Kyowa Hakko Kirin, Esai, Takeda Pharmaceutical, and Pfizer, and research funding from Chugai Pharmaceutical and AstraZeneca. SM has received remuneration from Pfizer and Yakult. SO has received remuneration from Chugai Pharmaceutical, AstraZeneca, Pfizer, Kyowa Hakko Kirin, and Eizai. MT has received remuneration from Novartis Pharma, Takeda Pharmaceutical, AstraZeneca, Taiho Pharmaceutical, Chugai Pharmaceutical, Pfizer, Eisai, Eli Lilly, Kyowa Hakko Kirin, and Genomic Health, has a consultant/advisory role at Genomic Health, and has received research funding from Taiho Pharmaceutical and Chugai Pharmaceutical. TM, CK, KK, HY, and HS have nothing to disclose.
Figures
References
-
- Perloff M, Lesnick GJ. Chemotherapy before and after mastectomy in stage III breast cancer. Arch Surg. 1982;117:879‐881. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

