eNOS deletion impairs mitochondrial quality control and exacerbates Western diet-induced NASH
- PMID: 31361543
- PMCID: PMC6842915
- DOI: 10.1152/ajpendo.00096.2019
eNOS deletion impairs mitochondrial quality control and exacerbates Western diet-induced NASH
Abstract
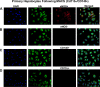
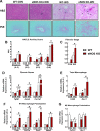
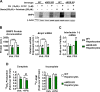
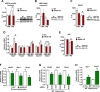
Dysregulated mitochondrial quality control leads to mitochondrial functional impairments that are central to the development and progression of hepatic steatosis to nonalcoholic steatohepatitis (NASH). Here, we identify hepatocellular localized endothelial nitric oxide synthase (eNOS) as a novel master regulator of mitochondrial quality control. Mice lacking eNOS were more susceptible to Western diet-induced hepatic inflammation and fibrosis in conjunction with decreased markers of mitochondrial biogenesis and turnover. The hepatocyte-specific influence was verified via magnetic activated cell sorting purified primary hepatocytes and in vitro siRNA-induced knockdown of eNOS. Hepatic mitochondria from eNOS knockout mice revealed decreased markers of mitochondrial biogenesis (PPARγ coactivator-1α, mitochondrial transcription factor A) and autophagy/mitophagy [BCL-2-interacting protein-3 (BNIP3), 1A/1B light chain 3B (LC3)], suggesting decreased mitochondrial turnover rate. eNOS knockout in primary hepatocytes exhibited reduced fatty acid oxidation capacity and were unable to mount a normal BNIP3 response to a mitophagic challenge compared with wild-type mice. Finally, we demonstrate that eNOS is required in primary hepatocytes to induce activation of the stress-responsive transcription factor nuclear factor erythroid 2-related factor 2 (NRF2). Thus, our data demonstrate that eNOS is an important regulator of hepatic mitochondrial content and function and NASH susceptibility.
Keywords: NAFLD; endothelial nitric oxide synthase; mitophagy; steatohepatitis.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


Similar articles
-
Critical Role for Hepatocyte-Specific eNOS in NAFLD and NASH.Diabetes. 2021 Nov;70(11):2476-2491. doi: 10.2337/db20-1228. Epub 2021 Aug 11. Diabetes. 2021. PMID: 34380696 Free PMC article.
-
Sex modulates hepatic mitochondrial adaptations to high-fat diet and physical activity.Am J Physiol Endocrinol Metab. 2019 Aug 1;317(2):E298-E311. doi: 10.1152/ajpendo.00098.2019. Epub 2019 Apr 30. Am J Physiol Endocrinol Metab. 2019. PMID: 31039007 Free PMC article.
-
Hepatic Mitochondrial Defects in a Nonalcoholic Fatty Liver Disease Mouse Model Are Associated with Increased Degradation of Oxidative Phosphorylation Subunits.Mol Cell Proteomics. 2018 Dec;17(12):2371-2386. doi: 10.1074/mcp.RA118.000961. Epub 2018 Aug 31. Mol Cell Proteomics. 2018. PMID: 30171159 Free PMC article.
-
Non-Alcoholic Fatty Liver Disease.Adv Exp Med Biol. 2017;960:443-467. doi: 10.1007/978-3-319-48382-5_19. Adv Exp Med Biol. 2017. PMID: 28585211 Review.
-
The Emerging Role of Hepatocellular eNOS in Non-alcoholic Fatty Liver Disease Development.Front Physiol. 2020 Jul 3;11:767. doi: 10.3389/fphys.2020.00767. eCollection 2020. Front Physiol. 2020. PMID: 32719616 Free PMC article. Review.
Cited by
-
Spirulina platensis Ameliorates Oxidative Stress Associated with Antiretroviral Drugs in HepG2 Cells.Plants (Basel). 2022 Nov 17;11(22):3143. doi: 10.3390/plants11223143. Plants (Basel). 2022. PMID: 36432871 Free PMC article.
-
Mitochondrial Dysfunction Plays Central Role in Nonalcoholic Fatty Liver Disease.Int J Mol Sci. 2022 Jun 30;23(13):7280. doi: 10.3390/ijms23137280. Int J Mol Sci. 2022. PMID: 35806284 Free PMC article. Review.
-
Hepatocyte-specific eNOS deletion impairs exercise-induced adaptations in hepatic mitochondrial function and autophagy.Obesity (Silver Spring). 2022 May;30(5):1066-1078. doi: 10.1002/oby.23414. Epub 2022 Mar 31. Obesity (Silver Spring). 2022. PMID: 35357089 Free PMC article.
-
Dysfunction of autophagy in high-fat diet-induced non-alcoholic fatty liver disease.Autophagy. 2024 Feb;20(2):221-241. doi: 10.1080/15548627.2023.2254191. Epub 2023 Sep 12. Autophagy. 2024. PMID: 37700498 Free PMC article. Review.
-
Mitochondrial metabolic dysfunction and non-alcoholic fatty liver disease: new insights from pathogenic mechanisms to clinically targeted therapy.J Transl Med. 2023 Jul 28;21(1):510. doi: 10.1186/s12967-023-04367-1. J Transl Med. 2023. PMID: 37507803 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

