The congenital myasthenic syndromes: expanding genetic and phenotypic spectrums and refining treatment strategies
- PMID: 31361628
- PMCID: PMC6735524
- DOI: 10.1097/WCO.0000000000000736
The congenital myasthenic syndromes: expanding genetic and phenotypic spectrums and refining treatment strategies
Abstract
Purpose of review: Congenital myasthenic syndromes (CMS) are a group of heterogeneous inherited disorders caused by mutations in genes encoding proteins whose function is essential for the integrity of neuromuscular transmission. This review updates the reader on the expanding phenotypic spectrum and suggested improved treatment strategies.
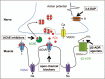
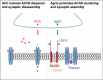
Recent findings: As next-generation sequencing is taken into the clinic, its use is both continuing to unearth new causative genes in which mutations underlie CMS and also broadening the phenotypic spectrum for known CMS genes. The number of genes in which mutations may cause neuromuscular transmission defects has now passed 30. The defective transmission may be part of an overall more complex phenotype in which there may be muscle, central nervous system or other involvement. Notably, mutations in series of genes encoding proteins located in the presynatic motor bouton have been identified. Rare cases of mutations in basal laminar proteins of the synaptic cleft are coming to light and additional mutations/phenotypic features have been located in some of the larger neuromuscular junction proteins such as AGRN and MUSK, where previously mutation screening by sanger sequencing was time consuming and costly. Finally, there are more reports of the beneficial effects of treatment with β2-adrenergic receptor agonists in patients, and the study of their action in disease models.
Summary: Recent studies of the CMS illustrate the increasing complexity of the genetics and pathophysiological mechanisms involved. With therapy tailored for the underlying disease mechanism treatment, although incomplete, is usually life-transforming. However, treatment for newly identified conditions in which myasthenia is only one component within complex multisystem disorder will prove challenging.
Figures

References
-
- Engel AG. Congenital myasthenic syndromes in 2018. Curr Neurol Neurosci Rep 2018; 18:46. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

