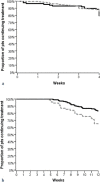
The influence of physiotherapy intervention on patients with multiple sclerosis-related spasticity treated with nabiximols (THC:CBD oromucosal spray)
- PMID: 31361750
- PMCID: PMC6667203
- DOI: 10.1371/journal.pone.0219670
The influence of physiotherapy intervention on patients with multiple sclerosis-related spasticity treated with nabiximols (THC:CBD oromucosal spray)
Abstract
Background: Nabiximols (THC/CBD Oromucosal Spray, Sativex) is used as an add-on therapy to treat moderate to severe spasticity of Multiple Sclerosis (MS).
Objectives: To examine the impact of physiotherapy (PT) programs on effectiveness and persistence of nabiximols treatment in people with MS-related spasticity.
Methods: This is an observational multicenter study with a follow-up period of 12 weeks, conducted in routine care settings in Italy. Patients with moderate to severe MS-related spasticity who started nabiximols were included. Spasticity was evaluated by the patient-rated 0-10 numerical rating scale (NRS). Clinical data were collected at baseline (T0), 4 weeks (T1) and 12 weeks (T2) months after enrollment.
Results: A total of 297 MS patients were selected, 290 completed the 3 months follow-up period. Mean NRS scores were 7.6 ± 1.1 at T0, 5.8 ± 1.4 at T1 and 5.5 ± 1.5 at T2. At T1, 77% of patients reached ≥20% improvement (initial response, IR); 22% reached ≥30% improvement (clinically relevant response, CRR). At T1, patients undergoing PT had a higher probability to reach CRR (Odds Ratio = 2.6 95% CI 1.3-5.6, p = 0.01). Nabiximols was discontinued in 30/290 (10.3%) patients at T1 (early discontinuers) and in 71/290 (24.5%) patients at T2 (late discontinuers). The probability of being late discontinuers was reduced in patients undergoing PT (Hazard Ratio = 0.41; 95% CI 0.23-0.69, p = 0.001).
Conclusions: Our real-life study confirms nabiximols' effectiveness in MS-related spasticity and suggests that the association of a PT program may improve overall response and persistence to nabiximols treatment.
Conflict of interest statement
De Giglio L received grants and personal fees from Novartis, Genzyme, Merck and Biogen. Haggiag S received speaking or travel fees from Biogen, Novartis, Genzyme, Eisai, CSL Behrig and Almirall. Monteleone F received personal fees or travel grants from Almirall, Biogen and Sanofi-Genzyme. Marfia G received personal fees from Almirall, Bayer Schering, Biogen Idec, Merck Serono, Novartis, Sanofi-Genzyme and Teva. Prosperini L received personal fees from Biogen, Teva, Almirall, Novartis, Genzyme, Roche and Merck Serono. Galgani S received personal fees from Biogen, Novartis, Genzyme, Almirall, Teva and Merck-Serono. Mirabella M received speaking fees, travel grants or research support from Almirall, Bayer Schering, Biogen, CSL Behring, Merck Serono, Novartis, Sanofi- Genzyme, Teva and Ultragenix. Centonze D received grants, personal fees or research support from Almirall, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Genzyme, Teva, Mitsubishi and Celgene. Pozzilli C reports grants and personal fees from Allergan, Almirall, Biogen, Genzyme, Hoffmann- La Roche Ltd, Merck Serono, Novartis, Sanofi and Teva. Castelli L received consulting fees from Almirall. The remaining authors have nothing to declare. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
Similar articles
-
Nabiximols (THC/CBD oromucosal spray, Sativex®) in clinical practice--results of a multicenter, non-interventional study (MOVE 2) in patients with multiple sclerosis spasticity.Eur Neurol. 2014;71(5-6):271-9. doi: 10.1159/000357427. Epub 2014 Feb 12. Eur Neurol. 2014. PMID: 24525548
-
Sativex® as add-on therapy vs. further optimized first-line ANTispastics (SAVANT) in resistant multiple sclerosis spasticity: a double-blind, placebo-controlled randomised clinical trial.Int J Neurosci. 2019 Feb;129(2):119-128. doi: 10.1080/00207454.2018.1481066. Epub 2018 Sep 13. Int J Neurosci. 2019. PMID: 29792372 Clinical Trial.
-
Sativex® (nabiximols) cannabinoid oromucosal spray in patients with resistant multiple sclerosis spasticity: the Belgian experience.BMC Neurol. 2021 Jun 22;21(1):227. doi: 10.1186/s12883-021-02246-0. BMC Neurol. 2021. PMID: 34157999 Free PMC article.
-
Review of Available Data for the Efficacy and Effectiveness of Nabiximols Oromucosal Spray (Sativex®) in Multiple Sclerosis Patients with Moderate to Severe Spasticity.Neurodegener Dis. 2021;21(3-4):55-62. doi: 10.1159/000520560. Epub 2021 Nov 3. Neurodegener Dis. 2021. PMID: 34731865 Review.
-
Evidence-based management of multiple sclerosis spasticity with nabiximols oromucosal spray in clinical practice: a 10-year recap.Neurodegener Dis Manag. 2022 Jun;12(3):141-154. doi: 10.2217/nmt-2022-0002. Epub 2022 Apr 4. Neurodegener Dis Manag. 2022. PMID: 35377770 Review.
Cited by
-
Efficacy of cannabinoids compared to the current standard treatments on symptom relief in persons with multiple sclerosis (CANSEP trial): study protocol for a randomized clinical trial.Front Neurol. 2024 Jul 24;15:1440678. doi: 10.3389/fneur.2024.1440678. eCollection 2024. Front Neurol. 2024. PMID: 39114536 Free PMC article.
-
A real-world evidence study of nabiximols in multiple sclerosis patients with resistant spasticity: Analysis in relation to the newly described 'spasticity-plus syndrome'.Eur J Neurol. 2022 Sep;29(9):2744-2753. doi: 10.1111/ene.15412. Epub 2022 Jun 7. Eur J Neurol. 2022. PMID: 35590453 Free PMC article.
-
Nabiximols and botulinum toxin injections for patients with multiple sclerosis: efficacy on spasticity and spasms in a single-centre experience.Neurol Sci. 2021 Dec;42(12):5037-5043. doi: 10.1007/s10072-021-05182-6. Epub 2021 Mar 19. Neurol Sci. 2021. PMID: 33742336
-
Cannabis and cannabinoids for symptomatic treatment for people with multiple sclerosis.Cochrane Database Syst Rev. 2022 May 5;5(5):CD013444. doi: 10.1002/14651858.CD013444.pub2. Cochrane Database Syst Rev. 2022. PMID: 35510826 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical