Jmjd6a regulates GSK3β RNA splicing in Xenopus laevis eye development
- PMID: 31361752
- PMCID: PMC6667200
- DOI: 10.1371/journal.pone.0219800
Jmjd6a regulates GSK3β RNA splicing in Xenopus laevis eye development
Abstract
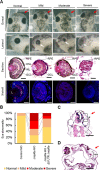
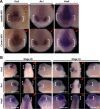
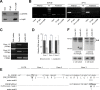
It has been suggested that Jmjd6 plays an important role in gene regulation through its demethylation or hydroxylation activity on histone and transcription factors. In addition, Jmjd6 has been shown to regulate RNA splicing by interaction with splicing factors. In this study, we demonstrated that Jmjd6a is expressed in developing Xenopus laevis eye during optic vesicle formation and retinal layer differentiation stages. Knockdown of Jmjd6a by an antisense morpholino resulted in eye malformation including a deformed retinal layer and no lens formation. We further found down-regulation of gene expression related to eye development such as Rx1, Otx2, and Pax6 in Jmjd6a morpholino injected embryos. Jmjd6 interacts with splicing factor U2AF25 and GSK3β RNA in the anterior region of Xenopus embryos. Knockdown of Jmjd6a led to deletion of GSK3β RNA exon 1 and 2, which resulted in generation of N'-terminal truncated GSK3β protein. This event further caused decreased phosphorylation of β-catenin and subsequently increased β-catenin stability. Therefore, our result may suggest that Jmjd6a plays an important role in Xenopus eye development through regulation of GSK3β RNA splicing and canonical Wnt/β-catenin signaling.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

