Acute changes of pro-inflammatory markers and corticosterone in experimental subarachnoid haemorrhage: A prerequisite for severity assessment
- PMID: 31361786
- PMCID: PMC6667150
- DOI: 10.1371/journal.pone.0220467
Acute changes of pro-inflammatory markers and corticosterone in experimental subarachnoid haemorrhage: A prerequisite for severity assessment
Abstract
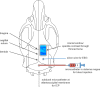
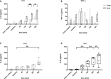
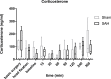
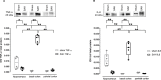
Many details of the pathophysiology of subarachnoid haemorrhage (SAH) still remain unknown, making animal experiments an indispensable tool for assessment of diagnostics and therapy. For animal protection and project authorization, one needs objective measures to evaluate the severity and burden in each model. Corticosterone is described as a sensitive stress parameter reflecting the acute burden, and inflammatory markers can be used for assessment of the extent of the brain lesion. However, the brain lesion itself may activate the hypothalamic-pituitary-adrenal-axis early after SAH, as shown for ischemic stroke, probably interfering with early inflammatory processes, thus complicating the assessment of severity and burden on the basis of corticosterone and inflammation. To assess the suitability of these markers in SAH, we evaluated the courses of corticosterone, IL-6 and TNF-α up to 6h in an acute model simulating SAH in continuously anaesthetized rats, lacking the pain and stress induced impact on these parameters. Animals were randomly allocated to sham or SAH. SAH was induced by cisterna magna blood-injection, and intracranial pressure and cerebral blood flow were measured under continuous isoflurane/fentanyl anaesthesia. Withdrawn at predetermined time points, blood was analysed by commercial ELISA kits. After 6h the brain was removed for western blot analysis of IL-6 and TNF-α. Serum corticosterone levels were low with no significant difference between sham and SAH. No activation of the HPA-axis was detectable, rendering corticosterone a potentially useful parameter for stress assessment in future chronic studies. Blood IL-6 and TNF-α increased in both groups over time, with IL-6 increasing significantly more in SAH compared to sham towards the end of the observation period. In the basal cortex, IL-6 and TNF-α increased only in SAH. The pro-inflammatory response seems to start locally in the brain, reflected by an increase in peripheral blood. An additional surgery-induced systemic inflammatory response should be considered.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

