Imbalance of autophagy and apoptosis in intestinal epithelium lacking the vitamin D receptor
- PMID: 31361973
- PMCID: PMC6902693
- DOI: 10.1096/fj.201900727R
Imbalance of autophagy and apoptosis in intestinal epithelium lacking the vitamin D receptor
Abstract
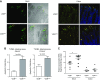
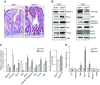
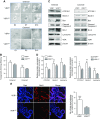
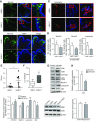
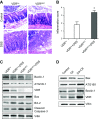
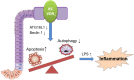
Apoptosis and autophagy are dynamic processes that determine the fate of cells. Vitamin D receptor (VDR) deficiency in the intestine leads to abnormal Paneth cells and impaired autophagy function. Here, we will elucidate the mechanisms of the intestinal epithelial VDR regulation of autophagy and apoptosis. We used in vivo VDRlox and VDR∆IEC mice and ex vivo organoids generated from small intestine and colon tissues. We found that VDR deficiency induced more apoptotic cells and significantly increased cell death in the small intestine and colon of VDR∆IEC mice. The proapoptotic protein B-cell lymphoma 2 (BCL-2) associated X protein (Bax) was enhanced, whereas autophagy related 16 like 1 (ATG16L1) and Beclin-1 were decreased in the intestines of VDRΔIEC mice. Apoptosis induced by Bax reduced autophagy by decreasing Beclin-1. Physical interactions between Beclin-1 and Bcl-2 were increased in the VDR-deficient epithelia from mice. The growth of VDR∆IEC organoids was significantly slower with fewer Paneth cells than that of VDR+/+ organoids. The expression levels of Beclin-1 and lysozyme were decreased in VDR∆IEC organoids. Bacterial endotoxin levels were high in the serum from VDR∆IEC mice and made mice susceptible to colitis. In the organoids and colitis IL-10-/- mice, vitamin D3 treatment increased VDR and ATG16L1 protein expression levels, which activated autophagic responses. In summary, intestinal epithelial VDR regulates autophagy and apoptosis through ATG16L1 and Beclin-1. Our studies provide fundamental insights into the tissue-specific function of VDR in modulating the balance between autophagy and apoptosis.-Lu, R., Zhang, Y.-G., Xia, Y., Sun, J. Imbalance of autophagy and apoptosis in intestinal epithelium lacking the vitamin D receptor.
Keywords: Beclin-1; VDR; colonoids; enteroids; inflammation.
Conflict of interest statement
The authors acknowledge U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK105118 and R01DK114126; U.S. Department of Defense Grant BC160450P1, Funding Opportunity W81XWH-17-1-0039; and the University of Illinois Cancer Center (to J.S.). R.L. is a recipient of the American Gastroenterological Association (AGA) Young Investigator Award, and orally presented some of these data at the Digestive Disease Week 2017. The authors declare no conflicts of interest.
Figures

Similar articles
-
Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis.Gut. 2015 Jul;64(7):1082-94. doi: 10.1136/gutjnl-2014-307436. Epub 2014 Jul 30. Gut. 2015. PMID: 25080448 Free PMC article.
-
Paneth Cell Alertness to Pathogens Maintained by Vitamin D Receptors.Gastroenterology. 2021 Mar;160(4):1269-1283. doi: 10.1053/j.gastro.2020.11.015. Epub 2020 Nov 18. Gastroenterology. 2021. PMID: 33217447 Free PMC article.
-
VDR/vitamin D receptor regulates autophagic activity through ATG16L1.Autophagy. 2016 Jun 2;12(6):1057-8. doi: 10.1080/15548627.2015.1072670. Epub 2015 Jul 28. Autophagy. 2016. PMID: 26218741 Free PMC article.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
-
Critical roles of intestinal epithelial vitamin D receptor signaling in controlling gut mucosal inflammation.J Steroid Biochem Mol Biol. 2015 Apr;148:179-83. doi: 10.1016/j.jsbmb.2015.01.011. Epub 2015 Jan 17. J Steroid Biochem Mol Biol. 2015. PMID: 25603468 Free PMC article. Review.
Cited by
-
Vitamin D/vitamin D receptor/Atg16L1 axis maintains podocyte autophagy and survival in diabetic kidney disease.Ren Fail. 2022 Dec;44(1):694-705. doi: 10.1080/0886022X.2022.2063744. Ren Fail. 2022. PMID: 35469547 Free PMC article.
-
Kazachstania pintolopesii in Blood and Intestinal Wall of Macrophage-Depleted Mice with Cecal Ligation and Puncture, the Control of Fungi by Macrophages during Sepsis.J Fungi (Basel). 2023 Dec 4;9(12):1164. doi: 10.3390/jof9121164. J Fungi (Basel). 2023. PMID: 38132765 Free PMC article.
-
Lipophilic and Hydrophilic Compounds from Arthrospira platensis and Its Effects on Tissue and Blood Cells-An Overview.Life (Basel). 2022 Sep 26;12(10):1497. doi: 10.3390/life12101497. Life (Basel). 2022. PMID: 36294932 Free PMC article. Review.
-
Ruscogenins Improve CD-Like Enteritis by Inhibiting Apoptosis of Intestinal Epithelial Cells and Activating Nrf2/NQO1 Pathway.Oxid Med Cell Longev. 2022 Mar 10;2022:4877275. doi: 10.1155/2022/4877275. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35308175 Free PMC article.
-
Effect of Vitamin D Deficiency and Supplementation in Lactation and Early Life on Allergic Airway Inflammation and the Expression of Autophagy-Related Genes in an Ovalbumin Mouse Model.J Inflamm Res. 2021 Aug 24;14:4125-4141. doi: 10.2147/JIR.S321642. eCollection 2021. J Inflamm Res. 2021. PMID: 34466017 Free PMC article.
References
-
- Shen L., Turner J. R. (2006) Role of epithelial cells in initiation and propagation of intestinal inflammation. Eliminating the static: tight junction dynamics exposed. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G577–G582 - PubMed
-
- Blikslager A. T., Moeser A. J., Gookin J. L., Jones S. L., Odle J. (2007) Restoration of barrier function in injured intestinal mucosa. Physiol. Rev. 87, 545–564 - PubMed
-
- Farhadi A., Banan A., Fields J., Keshavarzian A. (2003) Intestinal barrier: an interface between health and disease. J. Gastroenterol. Hepatol. 18, 479–497 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

