Salubrinal enhances eIF2α phosphorylation and improves fertility in a mouse model of Classic Galactosemia
- PMID: 31362041
- PMCID: PMC6751024
- DOI: 10.1016/j.bbadis.2019.07.010
Salubrinal enhances eIF2α phosphorylation and improves fertility in a mouse model of Classic Galactosemia
Abstract
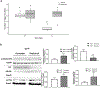
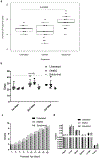
Loss of galactose-1 phosphate uridylyltransferase (GALT) activity in humans results in Classic Galactosemia, and the GalT-deficient (GalT-/-) mouse mimics the patient condition. GalT-/- ovaries display elevated endoplasmic reticulum (ER) stress marker, BiP, and downregulated canonical phosphatidylinositol 3-kinase (Pi3k)/protein kinase B (Akt) growth/pro-survival signaling. Numbers of primordial follicles are reduced in the mutants, recapitulating the accelerated ovarian aging seen in human patients. We previously found that oral administration of the compound Salubrinal (an eIF2α phosphatase inhibitor), resulted in reduction of ovarian BiP expression, rescued Pi3k/Akt signaling, and a doubling of primordial follicles in GalT-/- adults. Here, we further characterized galactosemic stress in GalT-/- mice versus wild-type (WT) controls, and examined whether Salubrinal treatment improved broader reproductive parameters. We assessed the expression levels of factors of the unfolded protein response (UPR), and found that BiP, phospho-Perk, and phospho-eIF2α were all elevated in GalT-/- ovaries. However, neither IKK activation (NFκB pathway) nor alternative Xbp1 splicing downstream of ER membrane protein Ire1α activation was induced, suggesting an Xbp1-independent UPR in galactosemic stress. Moreover, Salubrinal treatment significantly increased the number of ovulated eggs in mutant animals after gonadotrophic superovulation. Salubrinal treatment also normalized estrus cycle stage lengths and resulted in significantly larger litter sizes than vehicle-treated mutants. Overall, we show that Salubrinal protects against galactosemia-induced primordial follicle loss in a fashion that includes suppressing the de-phosphorylation of eIF2α, and that intervention in this way significantly improves and extends ovarian function, fertility, and fecundity.
Keywords: Classic Galactosemia; Galactosemic stress; Perk branch of the unfolded protein response (UPR); Pi3K/Akt signaling; Salubrinal; Subfertility.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures



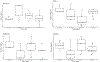
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

