Molecular Analysis of Canine Filaria and Its Wolbachia Endosymbionts in Domestic Dogs Collected from Two Animal University Hospitals in Bangkok Metropolitan Region, Thailand
- PMID: 31362350
- PMCID: PMC6789508
- DOI: 10.3390/pathogens8030114
Molecular Analysis of Canine Filaria and Its Wolbachia Endosymbionts in Domestic Dogs Collected from Two Animal University Hospitals in Bangkok Metropolitan Region, Thailand
Abstract
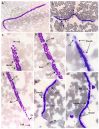
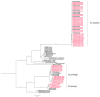
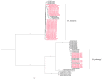
Canine filariasis is caused by several nematode species, such as Dirofilaria immitis, Dirofilaria repens, Brugia pahangi, Brugia malayi, and Acanthocheilonema reconditum. Zoonotic filariasis is one of the world's neglected tropical diseases. Since 2000, the World Health Organization (WHO) has promoted a global filarial eradication program to eliminate filariasis by 2020. Apart from vector control strategies, the infection control of reservoir hosts is necessary for more effective filariasis control. In addition, many studies have reported that Wolbachia is necessary for the development, reproduction, and survival of the filarial nematode. Consequently, the use of antibiotics to kill Wolbachia in nematodes has now become an alternative strategy to control filariasis. Previously, a case of subconjunctival dirofilariasis caused by Dirofilaria spp. has been reported in a woman who resides in the center of Bangkok, Thailand. Therefore, our study aimed to principally demonstrate the presence of filarial nematodes and Wolbachia bacteria in blood collected from domestic dogs from the Bangkok Metropolitan Region, Thailand. A total of 57 blood samples from dogs with suspected dirofilariasis who had visited veterinary clinics in Bangkok were collected. The investigations for the presence of microfilaria were carried out by using both microscopic and molecular examinations. PCR was used as the molecular detection method for the filarial nematodes based on the COI and ITS1 regions. The demonstration of Wolbachia was performed using PCR to amplify the FtsZ gene. All positive samples by PCR were then cloned and sequenced. The results showed that the filarial nematodes were detected in 16 samples (28.07%) using microscopic examinations. The molecular detection of filarial species using COI-PCR revealed that 50 samples (87.72%) were positive; these consisted of 33 (57.89%), 13 (22.81%), and 4 (7.02%) samples for D. immitis, B. pahangi, and B. malayi, respectively. While the ITS1-PCR showed that 41 samples (71.93%) were positive-30 samples (52.63%) were identified as containing D. immitis and 11 samples (19.30%) were identified to have B. pahangi, whereas B. malayi was not detected. Forty-seven samples (82.45%) were positive for Wolbachia DNA and the phylogenetic tree of all positive Wolbachia was classified into the supergroup C clade. This study has established fundamental data on filariasis associated with Wolbachia infection in domestic dogs in the Bangkok Metropolitan Region. An extensive survey of dog blood samples would provide valuable epidemiologic data on potential zoonotic filariasis in Thailand. In addition, this information could be used for the future development of more effective prevention and control strategies for canine filariasis in Thailand.
Keywords: B. malayi; B. pahangi; D. immitis; Thailand; dog; filariasis; zoonosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Genetic diversity and characterization of Wolbachia endosymbiont in canine filariasis.Acta Trop. 2023 Oct;246:107000. doi: 10.1016/j.actatropica.2023.107000. Epub 2023 Aug 9. Acta Trop. 2023. PMID: 37567493
-
Detection of filarial parasites in domestic cats by PCR-RFLP of ITS1.Vet Parasitol. 2006 Sep 10;140(3-4):366-72. doi: 10.1016/j.vetpar.2006.04.003. Epub 2006 May 19. Vet Parasitol. 2006. PMID: 16713099
-
Development of a multiplex qPCR-based approach for the diagnosis of Dirofilaria immitis, D. repens and Acanthocheilonema reconditum.Parasit Vectors. 2020 Jun 22;13(1):319. doi: 10.1186/s13071-020-04185-0. Parasit Vectors. 2020. PMID: 32571427 Free PMC article.
-
Wolbachia in filarial nematodes: evolutionary aspects and implications for the pathogenesis and treatment of filarial diseases.Vet Parasitol. 2001 Jul 12;98(1-3):215-38. doi: 10.1016/s0304-4017(01)00432-0. Vet Parasitol. 2001. PMID: 11516587 Review.
-
Endosymbiotic Wolbachia of parasitic filarial nematodes as drug targets.Indian J Med Res. 2005 Sep;122(3):199-204. Indian J Med Res. 2005. PMID: 16251775 Review.
Cited by
-
First study on microscopic and molecular detection of Acanthocheilonema reconditum and Leishmania infantum coinfection in dogs in Southwest Colombia.Vet World. 2023 Jan;16(1):94-103. doi: 10.14202/vetworld.2023.94-103. Epub 2023 Jan 12. Vet World. 2023. PMID: 36855357 Free PMC article.
-
Emergence of Dirofilaria repens (Spirurida: Onchocercidae) in dogs in Eastern Thailand.Vet World. 2021 Nov;14(11):2851-2854. doi: 10.14202/vetworld.2021.2851-2854. Epub 2021 Nov 6. Vet World. 2021. PMID: 35017830 Free PMC article.
-
Ocular Brugia pahangi Filariasis Complicated by Severe Macular Damage in Thailand: Case Report and Literature Review.Am J Trop Med Hyg. 2024 Apr 30;110(6):1158-1164. doi: 10.4269/ajtmh.24-0047. Print 2024 Jun 5. Am J Trop Med Hyg. 2024. PMID: 38688273 Free PMC article. Review.
-
Molecular detection of Dirofilaria immitis and its Wolbachia endosymbionts in dogs from Myanmar.Curr Res Parasitol Vector Borne Dis. 2023 Oct 4;4:100148. doi: 10.1016/j.crpvbd.2023.100148. eCollection 2023. Curr Res Parasitol Vector Borne Dis. 2023. PMID: 38021190 Free PMC article.
-
New Molecular Data on Filaria and its Wolbachia from Red Howler Monkeys (Alouatta macconnelli) in French Guiana-A Preliminary Study.Pathogens. 2020 Jul 31;9(8):626. doi: 10.3390/pathogens9080626. Pathogens. 2020. PMID: 32752052 Free PMC article.
References
-
- World Health Organization Lymphatic Filariasis. [(accessed on 28 May 2019)]; Available online: https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis.
-
- Kaikuntod M., Thongkorn K., Tiwananthagorn S., Boonyapakorn C. Filarial worms in dogs in Southeast Asia. Vet. Integr. Sci. 2018;16:1–17.
-
- Mak J.W., Yen P.K., Lim K.C., Ramiah N. Zoonotic implications of cats and dogs in filarial transmission in Peninsular Malaysia. Trop. Geogr. Med. 1980;32:259–264. - PubMed
-
- Kelly J.D. Current Veterinary Therapy VII. W. B. Saunders; Philadelphia, PA, USA: 1979. Canine heart worm disease; pp. 326–335.
LinkOut - more resources
Full Text Sources
Miscellaneous

