Cell Intrinsic and Extrinsic Mechanisms of Caveolin-1-Enhanced Metastasis
- PMID: 31362353
- PMCID: PMC6723107
- DOI: 10.3390/biom9080314
Cell Intrinsic and Extrinsic Mechanisms of Caveolin-1-Enhanced Metastasis
Abstract
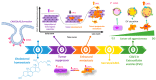
Caveolin-1 (CAV1) is a scaffolding protein with a controversial role in cancer. This review will initially discuss earlier studies focused on the role as a tumor suppressor before elaborating subsequently on those relating to function of the protein as a promoter of metastasis. Different mechanisms are summarized illustrating how CAV1 promotes such traits upon expression in cancer cells (intrinsic mechanisms). More recently, it has become apparent that CAV1 is also a secreted protein that can be included into exosomes where it plays a significant role in determining cargo composition. Thus, we will also discuss how CAV1 containing exosomes from metastatic cells promote malignant traits in more benign recipient cells (extrinsic mechanisms). This ability appears, at least in part, attributable to the transfer of specific cargos present due to CAV1 rather than the transfer of CAV1 itself. The evolution of how our perception of CAV1 function has changed since its discovery is summarized graphically in a time line figure.
Keywords: caveolae; cholesterol transport; exosomes; metastasis promoter; tumor suppressor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Root K.T., Plucinsky S.M., Glover K.J. Recent progress in the topology, structure, and oligomerization of caveolin: A building block of caveolae. Curr. Top. Membr. 2015;75:305–336. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

