Expression of HERV Genes as Possible Biomarker and Target in Neurodegenerative Diseases
- PMID: 31362360
- PMCID: PMC6696274
- DOI: 10.3390/ijms20153706
Expression of HERV Genes as Possible Biomarker and Target in Neurodegenerative Diseases
Abstract
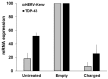
Human endogenous retroviruses (HERVs) are genetic parasites, in-between genetics and environment. Few HERVs retain some coding capability. Sometimes, the host has the advantage of some HERV genes; conversely, HERVs may contribute to pathogenesis. The expression of HERVs depends on several factors, and is regulated epigenetically by stimuli such as inflammation, viral and microbial infections, etc. Increased expression of HERVs occurs in physiological and pathological conditions, in one or more body sites. Several diseases have been attributed to one or more HERVs, particularly neurological diseases. The key problem is to differentiate the expression of a HERV as cause or effect of a disease. To be used as a biomarker, a correlation between the expression of a certain HERV and the disease onset and/or behavior must be found. The greater challenge is to establish a pathogenic role. The criteria defining causal connections between HERVs and diseases include the development of animal models, and disease modulation in humans, by anti-HERV therapeutic antibody. So far, statistically significant correlations between HERVs and diseases have been achieved for HERV-W and multiple sclerosis; disease reproduction in transgenic animals was achieved for HERV-W and multiple sclerosis, and for HERV-K and amyotrophic lateral sclerosis. Clinical trials for both diseases are in progress.
Keywords: HERV human endogenous retroviruses; HERV-Kenv; HERV-Wenv; TDP-43; amyotrophic lateral sclerosis; multiple sclerosis; neuroAIDS; neurodegeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

