Arctium lappa Extract Suppresses Inflammation and Inhibits Melanoma Progression
- PMID: 31362372
- PMCID: PMC6789568
- DOI: 10.3390/medicines6030081
Arctium lappa Extract Suppresses Inflammation and Inhibits Melanoma Progression
Abstract
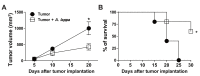
Background: Arctium lappa has been used as popular medicinal herb and health supplement in Chinese societies. Bioactive components from A. lappa have attracted the attention of researchers due to their promising therapeutic effects. In this study, we investigated the effects of A. lappa hydroalcoholic extract (Alhe) during different models of inflammation, in vivo. Methods: The anti-inflammatory activity was evaluated through the air pouch model. For this, mice received an inflammatory stimulus with lipopolysaccharide (LPS) and were later injected with Alhe. To assess anti-tumoral activity, the animals were inoculated with B16F10 cells and injected with Alhe every 5 days, along the course of 30 days. Controls were submitted to the same conditions and injected with the vehicle. Peritoneal or air pouch fluids were collected to evaluate leukocyte counting or cellular activation via quantification of cytokines and nitric oxide. Results: Alhe injection reduced the neutrophil influx and production of inflammatory mediators in inflammatory foci after LPS or tumor challenges. Furthermore, Alhe injection reduced tumor growth and enhanced mice survival. Conclusions: Collectively, these data suggest that Alhe regulates immune cell migration and activation, which correlates with favorable outcome in mouse models of acute inflammation and melanoma progression.
Keywords: Arctium lappa; inflammation; melanoma; natural product; phytomedicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Harnessing the power of Arctium lappa root: a review of its pharmacological properties and therapeutic applications.Nat Prod Bioprospect. 2024 Aug 20;14(1):49. doi: 10.1007/s13659-024-00466-8. Nat Prod Bioprospect. 2024. PMID: 39162715 Free PMC article. Review.
-
Overview of the anti-inflammatory effects, pharmacokinetic properties and clinical efficacies of arctigenin and arctiin from Arctium lappa L.Acta Pharmacol Sin. 2018 May;39(5):787-801. doi: 10.1038/aps.2018.32. Epub 2018 Apr 26. Acta Pharmacol Sin. 2018. PMID: 29698388 Free PMC article. Review.
-
Natural Arctium lappa fruit extract improves the clinical signs of aging skin.J Cosmet Dermatol. 2008 Dec;7(4):281-9. doi: 10.1111/j.1473-2165.2008.00407.x. J Cosmet Dermatol. 2008. PMID: 19146605 Clinical Trial.
-
Use of Arctium lappa Extract Against Acetaminophen-Induced Hepatotoxicity in Rats.Curr Ther Res Clin Exp. 2015 May 27;77:73-8. doi: 10.1016/j.curtheres.2015.05.001. eCollection 2015 Dec. Curr Ther Res Clin Exp. 2015. PMID: 26543508 Free PMC article.
-
Arctium lappa L. root extract induces cell death via mitochondrial-mediated caspase-dependent apoptosis in Jurkat human leukemic T cells.Biomed Pharmacother. 2019 Feb;110:918-929. doi: 10.1016/j.biopha.2018.12.023. Epub 2018 Dec 17. Biomed Pharmacother. 2019. PMID: 30572196
Cited by
-
Harnessing the power of Arctium lappa root: a review of its pharmacological properties and therapeutic applications.Nat Prod Bioprospect. 2024 Aug 20;14(1):49. doi: 10.1007/s13659-024-00466-8. Nat Prod Bioprospect. 2024. PMID: 39162715 Free PMC article. Review.
-
Green Propolis Compounds (Baccarin and p-Coumaric Acid) Show Beneficial Effects in Mice for Melanoma Induced by B16f10.Medicines (Basel). 2021 Apr 30;8(5):20. doi: 10.3390/medicines8050020. Medicines (Basel). 2021. PMID: 33946188 Free PMC article.
-
Evaluation of antimicrobial and cytotoxic effects of Echinacea and Arctium extracts and Zataria essential oil.AMB Express. 2022 Jun 15;12(1):75. doi: 10.1186/s13568-022-01417-7. AMB Express. 2022. PMID: 35705727 Free PMC article.
-
Use of Medicinal Plants in the Process of Wound Healing: A Literature Review.Pharmaceuticals (Basel). 2024 Feb 27;17(3):303. doi: 10.3390/ph17030303. Pharmaceuticals (Basel). 2024. PMID: 38543089 Free PMC article. Review.
-
Arctium lappa Root Extract Prevents Lead-Induced Liver Injury by Attenuating Oxidative Stress and Inflammation, and Activating Akt/GSK-3β Signaling.Antioxidants (Basel). 2019 Nov 24;8(12):582. doi: 10.3390/antiox8120582. Antioxidants (Basel). 2019. PMID: 31771282 Free PMC article.
References
LinkOut - more resources
Full Text Sources

