An Omics Approach to Extracellular Vesicles from HIV-1 Infected Cells
- PMID: 31362387
- PMCID: PMC6724219
- DOI: 10.3390/cells8080787
An Omics Approach to Extracellular Vesicles from HIV-1 Infected Cells
Abstract
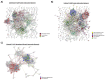
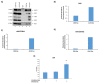
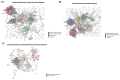
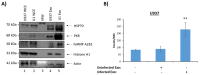
Human Immunodeficiency Virus-1 (HIV-1) is the causative agent of Acquired Immunodeficiency Syndrome (AIDS), infecting nearly 37 million people worldwide. Currently, there is no definitive cure, mainly due to HIV-1's ability to enact latency. Our previous work has shown that exosomes, a small extracellular vesicle, from uninfected cells can activate HIV-1 in latent cells, leading to increased mostly short and some long HIV-1 RNA transcripts. This is consistent with the notion that none of the FDA-approved antiretroviral drugs used today in the clinic are transcription inhibitors. Furthermore, these HIV-1 transcripts can be packaged into exosomes and released from the infected cell. Here, we examined the differences in protein and nucleic acid content between exosomes from uninfected and HIV-1-infected cells. We found increased cyclin-dependent kinases, among other kinases, in exosomes from infected T-cells while other kinases were present in exosomes from infected monocytes. Additionally, we found a series of short antisense HIV-1 RNA from the 3' LTR that appears heavily mutated in exosomes from HIV-1-infected cells along with the presence of cellular noncoding RNAs and cellular miRNAs. Both physical and functional validations were performed on some of the key findings. Collectively, our data indicate distinct differences in protein and RNA content between exosomes from uninfected and HIV-1-infected cells, which can lead to different functional outcomes in recipient cells.
Keywords: HIV-1; RNA sequencing; extracellular vesicle; proteomics.
Conflict of interest statement
None of the authors have conflicts of interest to disclose.
Figures



References
-
- WHO|World Health Organization. [(accessed on 26 April 2017)]; Available online: http://www.who.int/hiv/en/
-
- HIV in the United States|Statistics Overview|Statistics Center|HIV/AIDS|CDC. [(accessed on 26 April 2017)]; Available online: https://www.cdc.gov/hiv/statistics/overview/ataglance.html.
-
- U.S. Statistics|HIV.gov. [(accessed on 4 January 2019)]; Available online: https://www.hiv.gov/hiv-basics/overview/data-and-trends/statistics.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

