Aristoteline, an Indole-Alkaloid, Induces Relaxation by Activating Potassium Channels and Blocking Calcium Channels in Isolated Rat Aorta
- PMID: 31362388
- PMCID: PMC6695676
- DOI: 10.3390/molecules24152748
Aristoteline, an Indole-Alkaloid, Induces Relaxation by Activating Potassium Channels and Blocking Calcium Channels in Isolated Rat Aorta
Abstract
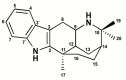
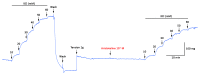
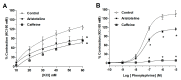
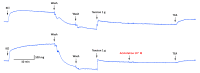
Alkaloids derived from plants have shown great medicinal benefits, and are often reported for their use in cardiovascular disease management. Aristotelia chilensis (Molina) Stuntz (Maqui) has shown important medicinal properties in traditional useage. In this study, we evaluated the effect of the indole-alkaloid aristoteline (ARI), isolated from leaves of Maqui, on vascular reactivity of isolated aortic rings from normotensive rats. ARI induced relaxation (100%) in a concentration-dependent manner in intact or denuded-endothelium aortic rings pre-contracted with phenylephrine (PE; 1 μM). However, a specific soluble guanylyl cyclase inhibitor (ODQ; 1 μM) significantly reduced the relaxation to ARI in aortic rings pre-contracted with PE. In the presence of ARI, the contraction induced by KCl or PE was significantly (p < 0.05) decreased. Interestingly, the potassium channel blockade with 10 μM BaCl2 (Kir), 10 μM glibenclamide (KATP), 1 mM tetraethylammonium (TEA; KCa1.1), or 1 mM 4-aminopyridine (4-AP; Kv) significantly (p < 0.05) reduced the ARI-induced relaxation. ARI significantly (p < 0.05) reduced the contractile response to agonist of CaV1.2 channels (Bay K8644; 10 nM), likely reducing the influx of extracellular calcium through plasma membrane. The mechanisms associated with this process suggest an activation of the potassium channels, a calcium-induced antagonism and endothelium independent vasodilation that possibly involves the nitric oxide-independent soluble guanylate cyclase pathway.
Keywords: Aristoteline; calcium channels; potassium channels; rat; vascular activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Zuniga G.E., Tapia A., Arenas A., Contreras R.A., Zuniga-Libano G. Phytochemistry and biological properties of Aristotelia chilensis a Chilean blackberry: a review. Phytochem. Rev. 2017;16:1081–1094. doi: 10.1007/s11101-017-9533-1. - DOI
-
- Rodríguez K., Ah-Hen K.S., Vega-Gálvez A., Vásquez V., Quispe-Fuentes I., Rojas P., Lemus-Mondaca R. Changes in bioactive components and antioxidant capacity of maqui, Aristotelia chilensis [Mol] Stuntz, berries during drying. LWT Food Sci. Technol. 2016;65:537–542. doi: 10.1016/j.lwt.2015.08.050. - DOI
-
- Cespedes C.L., Pavon N., Dominguez M., Alarcon J., Balbontin C., Kubo I., El-Hafidi M., Avila J.G. The chilean superfruit black-berry Aristotelia chilensis (Elaeocarpaceae), Maqui as mediator in inflammation-associated disorders. Food Chem. Toxicol. 2017;108:438–450. doi: 10.1016/j.fct.2016.12.036. - DOI - PubMed
-
- Munoz O., Christen P., Cretton S., Backhouse N., Torres V., Correa O., Costa E., Miranda H., Delporte C. Chemical study and anti-inflammatory, analgesic and antioxidant activities of the leaves of Aristotelia chilensis (Mol.) Stuntz, Elaeocarpaceae. J. Pharm. Pharmacol. 2011;63:849–859. doi: 10.1111/j.2042-7158.2011.01280.x. - DOI - PubMed
-
- Cespedes C.L., Balbontin C., Avila J.G., Dominguez M., Alarcon J., Paz C., Burgos V., Ortiz L., Penaloza-Castro I., Seigler D.S., et al. Inhibition on cholinesterase and tyrosinase by alkaloids and phenolics from Aristotelia chilensis leaves. Food Chem. Toxicol. 2017;109:984–995. doi: 10.1016/j.fct.2017.05.009. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

