Lutathera®: The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy
- PMID: 31362406
- PMCID: PMC6789871
- DOI: 10.3390/ph12030114
Lutathera®: The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy
Abstract
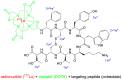
As the first radiopharmaceutical for Peptide Receptor Radionuclide Therapy (PRRT), Lutathera® was approved by the EMA in 2017 and the FDA in 2018 for the treatment of somatostatin receptor (SSTR) positive gastroenteropancreatic neuroendocrine tumors. Using the concept of PRRT, Lutathera® combines the radionuclide 177Lu with the somatostatin analogue DOTA-TATE, thus delivering ionizing radiation specifically to tumor cells expressing somatostatin receptors. As a result, DNA single- and double-strand breaks are provoked, in case of double-strand breaks leading to cell death of the tumor and its SSTR-positive lesions.
Keywords: Lutathera®, [177Lu]Lu-DOTA-TATE; neuroendocrine tumors (NET); peptide receptor radionuclide therapy (PRRT); somatostatin receptor (SSTR); thera(g)nostics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Coenen H.H., Gee A.D., Adam M., Antoni G., Cutler C.S., Fujibayashi Y., Jeong J.M., Mach R.H., Mindt T.L., Pike V.W., et al. Open letter to journal editors: International consensus radiochemistry nomenclature guidelines. J. Label. Compd. Radiopharm. 2018;61:402–404. doi: 10.1002/jlcr.3604. - DOI - PubMed
-
- FDA Letter of Approval for LUTATHERA®. [(accessed on 23 April 2019)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2018/208700Orig....
-
- Authorization details for Lutathera® in Europe. [(accessed on 23 April 2019)]; Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/lutathera#authorisatio....
-
- Authorization details for Lutathera® in Canada. [(accessed on 23 April 2019)]; Available online: https://hpr-rps.hres.ca/reg-content/regulatory-decision-summary-detail.p....
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous