Bottom-Up Proteomic Analysis of Polypeptide Venom Components of the Giant Ant Dinoponera Quadriceps
- PMID: 31362422
- PMCID: PMC6722740
- DOI: 10.3390/toxins11080448
Bottom-Up Proteomic Analysis of Polypeptide Venom Components of the Giant Ant Dinoponera Quadriceps
Abstract
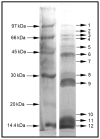
Ant species have specialized venom systems developed to sting and inoculate a biological cocktail of organic compounds, including peptide and polypeptide toxins, for the purpose of predation and defense. The genus Dinoponera comprises predatory giant ants that inoculate venom capable of causing long-lasting local pain, involuntary shaking, lymphadenopathy, and cardiac arrhythmias, among other symptoms. To deepen our knowledge about venom composition with regard to protein toxins and their roles in the chemical-ecological relationship and human health, we performed a bottom-up proteomics analysis of the crude venom of the giant ant D. quadriceps, popularly known as the "false" tocandiras. For this purpose, we used two different analytical approaches: (i) gel-based proteomics approach, wherein the crude venom was resolved by denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and all protein bands were excised for analysis; (ii) solution-based proteomics approach, wherein the crude venom protein components were directly fragmented into tryptic peptides in solution for analysis. The proteomic data that resulted from these two methodologies were compared against a previously annotated transcriptomic database of D. quadriceps, and subsequently, a homology search was performed for all identified transcript products. The gel-based proteomics approach unequivocally identified nine toxins of high molecular mass in the venom, as for example, enzymes [hyaluronidase, phospholipase A1, dipeptidyl peptidase and glucose dehydrogenase/flavin adenine dinucleotide (FAD) quinone] and diverse venom allergens (homologous of the red fire ant Selenopsis invicta) and venom-related proteins (major royal jelly-like). Moreover, the solution-based proteomics revealed and confirmed the presence of several hydrolases, oxidoreductases, proteases, Kunitz-like polypeptides, and the less abundant inhibitor cysteine knot (ICK)-like (knottin) neurotoxins and insect defensin. Our results showed that the major components of the D. quadriceps venom are toxins that are highly likely to damage cell membranes and tissue, to cause neurotoxicity, and to induce allergic reactions, thus, expanding the knowledge about D. quadriceps venom composition and its potential biological effects on prey and victims.
Keywords: Dinoponera quadriceps; Formicidae; Hymenoptera venom; ICK-like toxins; proteomics; venom allergens.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study.
Figures










Similar articles
-
Transcriptome analysis in venom gland of the predatory giant ant Dinoponera quadriceps: insights into the polypeptide toxin arsenal of hymenopterans.PLoS One. 2014 Jan 31;9(1):e87556. doi: 10.1371/journal.pone.0087556. eCollection 2014. PLoS One. 2014. PMID: 24498135 Free PMC article.
-
Peptidomic comparison and characterization of the major components of the venom of the giant ant Dinoponera quadriceps collected in four different areas of Brazil.J Proteomics. 2013 Dec 6;94:413-22. doi: 10.1016/j.jprot.2013.10.017. Epub 2013 Oct 22. J Proteomics. 2013. PMID: 24157790
-
Comprehensive analysis of peptides and low molecular weight components of the giant ant Dinoponera quadriceps venom.Biol Chem. 2020 Jul 28;401(8):945-954. doi: 10.1515/hsz-2019-0397. Biol Chem. 2020. PMID: 32229648
-
[Ants: a chemical library of anticancer molecules].Biol Aujourdhui. 2016;210(2):119-25. doi: 10.1051/jbio/2016021. Epub 2016 Sep 30. Biol Aujourdhui. 2016. PMID: 27687602 Review. French.
-
The Biochemical Toxin Arsenal from Ant Venoms.Toxins (Basel). 2016 Jan 20;8(1):30. doi: 10.3390/toxins8010030. Toxins (Basel). 2016. PMID: 26805882 Free PMC article. Review.
Cited by
-
Investigation of the structure-activity relationship in ponericin L1 from Neoponera goeldii.Pept Sci (Hoboken). 2020 May;112(3):e24162. doi: 10.1002/pep2.24162. Epub 2020 Mar 31. Pept Sci (Hoboken). 2020. PMID: 33937618 Free PMC article.
-
Social complexity, life-history and lineage influence the molecular basis of castes in vespid wasps.Nat Commun. 2023 Feb 24;14(1):1046. doi: 10.1038/s41467-023-36456-6. Nat Commun. 2023. PMID: 36828829 Free PMC article.
-
Proteo-Trancriptomic Analyses Reveal a Large Expansion of Metalloprotease-Like Proteins in Atypical Venom Vesicles of the Wasp Meteorus pulchricornis (Braconidae).Toxins (Basel). 2021 Jul 19;13(7):502. doi: 10.3390/toxins13070502. Toxins (Basel). 2021. PMID: 34357975 Free PMC article.
-
Antifungal In Vitro Activity of Pilosulin- and Ponericin-Like Peptides from the Giant Ant Dinoponera quadriceps and Synergistic Effects with Antimycotic Drugs.Antibiotics (Basel). 2020 Jun 23;9(6):354. doi: 10.3390/antibiotics9060354. Antibiotics (Basel). 2020. PMID: 32585881 Free PMC article.
-
Shedding Lights on Crude Venom from Solitary Foraging Predatory Ant Ectatomma opaciventre: Initial Toxinological Investigation.Toxins (Basel). 2022 Jan 4;14(1):37. doi: 10.3390/toxins14010037. Toxins (Basel). 2022. PMID: 35051015 Free PMC article.
References
-
- Wilson R., Gullan P.J., Cranston P.S. The Insects: An Outline of Entomology. 4th ed. Volume 14 Wiley; Hoboken, NJ, USA: 2010.
-
- Fernández F., Ospina M. Sinopsis de las Hormigas de la Región Neotropical. Instituto de Investigación de Recursos Biológicos Alexander Von Humbolt; Bogotá, Colombia: 2003.
-
- Schoeters E., Billen J. Morphology and ultrastructure of the convoluted gland in the ant Dinoponera australis (Hymenoptera: Formicidae) Int. J. Insect Morphol. Embryol. 1995;24:323–332. doi: 10.1016/0020-7322(94)00024-K. - DOI
-
- Robinson S.D., Mueller A., Clayton D., Starobova H., Hamilton B.R., Payne R.J., Vetter I., King G.F., Undheim E.A.B. A comprehensive portrait of the venom of the giant red bull ant, Myrmecia gulosa, reveals a hyperdiverse hymenopteran toxin gene family. Sci. Adv. 2018;4:eaau4640. doi: 10.1126/sciadv.aau4640. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

