Serological inference of past primary and secondary dengue infection: implications for vaccination
- PMID: 31362614
- PMCID: PMC6685028
- DOI: 10.1098/rsif.2019.0207
Serological inference of past primary and secondary dengue infection: implications for vaccination
Abstract
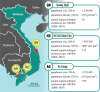
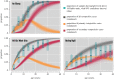
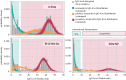
Owing to the finding that Dengvaxia® (the only licensed dengue vaccine to date) increases the risk of severe illness among seronegative recipients, the World Health Organization has recommended screening individuals for their serostatus prior to vaccination. To decide whether and how to carry out screening, it is necessary to estimate the transmission intensity of dengue and to understand the performance of the screening method. In this study, we inferred the annual force of infection (FOI; a measurement of transmission intensity) of dengue virus in three locations in Vietnam: An Giang (FOI = 0.04 for the below 10 years age group and FOI = 0.20 for the above 10 years age group), Ho Chi Minh City (FOI = 0.12) and Quang Ngai (FOI = 0.05). In addition, we show that using a quantitative approach to immunoglobulin G (IgG) levels (measured by indirect enzyme-linked immunosorbent assays) can help to distinguish individuals with primary exposures (primary seropositive) from those with secondary exposures (secondary seropositive). We found that primary-seropositive individuals-the main targets of the vaccine-tend to have a lower IgG level, and, thus, they have a higher chance of being misclassified as seronegative than secondary-seropositive cases. However, screening performance can be improved by incorporating patient age and transmission intensity into the interpretation of IgG levels.
Keywords: IgG antibody; Vietnam; dengue; force of infection; serostatus; vaccination.
Conflict of interest statement
We declare that we have no competing interests.
Figures

Similar articles
-
Estimating the force of infection of four dengue serotypes from serological studies in two regions of Vietnam.PLoS Negl Trop Dis. 2024 Oct 7;18(10):e0012568. doi: 10.1371/journal.pntd.0012568. eCollection 2024 Oct. PLoS Negl Trop Dis. 2024. PMID: 39374298 Free PMC article.
-
Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy.N Engl J Med. 2018 Jul 26;379(4):327-340. doi: 10.1056/NEJMoa1800820. Epub 2018 Jun 13. N Engl J Med. 2018. PMID: 29897841
-
Optimal dengue vaccination strategies of seropositive individuals.Math Biosci Eng. 2019 Feb 15;16(3):1171-1189. doi: 10.3934/mbe.2019056. Math Biosci Eng. 2019. PMID: 30947414
-
The first licensed dengue vaccine: can it be used in travelers?Curr Opin Infect Dis. 2019 Oct;32(5):394-400. doi: 10.1097/QCO.0000000000000573. Curr Opin Infect Dis. 2019. PMID: 31305495 Review.
-
Dengue vaccine development: status and future.Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2020 Jan;63(1):40-44. doi: 10.1007/s00103-019-03060-3. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2020. PMID: 31784763 Free PMC article. Review.
Cited by
-
Age-dependent heterogeneity in the antigenic effects of mutations to influenza hemagglutinin.Cell Host Microbe. 2024 Aug 14;32(8):1397-1411.e11. doi: 10.1016/j.chom.2024.06.015. Epub 2024 Jul 19. Cell Host Microbe. 2024. PMID: 39032493 Free PMC article.
-
Reconciling heterogeneous dengue virus infection risk estimates from different study designs.Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2411768121. doi: 10.1073/pnas.2411768121. Epub 2024 Dec 31. Proc Natl Acad Sci U S A. 2025. PMID: 39739790 Free PMC article.
-
Comparative Analysis of Long-Term Measles Immune Response After Natural Infection and Routine Vaccination in China.Vaccines (Basel). 2025 May 23;13(6):555. doi: 10.3390/vaccines13060555. Vaccines (Basel). 2025. PMID: 40573886 Free PMC article.
-
Serodynamics: A primer and synthetic review of methods for epidemiological inference using serological data.Epidemics. 2024 Dec;49:100806. doi: 10.1016/j.epidem.2024.100806. Epub 2024 Nov 30. Epidemics. 2024. PMID: 39647462 Free PMC article. Review.
-
Seroprotection against tetanus in southern Vietnam.Vaccine. 2023 Mar 24;41(13):2208-2213. doi: 10.1016/j.vaccine.2023.02.036. Epub 2023 Feb 26. Vaccine. 2023. PMID: 36849339 Free PMC article.
References
-
- World Health Organization. 2019. Dengue and severe dengue. https://www.who.int/en/news-room/fact-sheets/detail/dengue-and-severe-de... (accessed on 13 May 2019).
-
- World Health Organization. 2019. Dengue. http://www.searo.who.int/entity/vector_borne_tropical_diseases/data/data... (accessed 13 May 2019).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

