Dysregulation of lncRNA and circRNA Expression in Mouse Testes after Exposure to Triptolide
- PMID: 31362668
- PMCID: PMC7062010
- DOI: 10.2174/1389200220666190729130020
Dysregulation of lncRNA and circRNA Expression in Mouse Testes after Exposure to Triptolide
Abstract
Background: Triptolide has been shown to exert various pharmacological effects on systemic autoimmune diseases and cancers. However, its severe toxicity, especially reproductive toxicity, prevents its widespread clinical use for people with fertility needs. Noncoding RNAs including lncRNAs and circRNAs are novel regulatory molecules that mediate a wide variety of physiological activities; they are crucial for spermatogenesis and their dysregulation might cause male infertility. However, whether they are involved in triptolide-induced reproductive toxicity is completely unknown.
Methods: After exposure of mice to triptolide, the total RNAs were used to investigate lncRNA/circRNA/mRNA expression profiles by strand-specific RNA sequencing at the transcriptome level to help uncover RNA-related mechanisms in triptolide-induced toxicity.
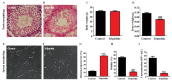
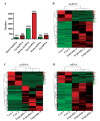
Results: Triptolide significantly decreased testicular weight, damaged testis and sperm morphology, and reduced sperm motility and density. Remarkable deformities in sperm head and tail were also found in triptolide-exposed mice. At the transcriptome level, the triptolide-treated mice exhibited aberrant expression profiles of lncRNAs/circRNAs/mRNAs. Gene Ontology and pathway analyses revealed that the functions of the differentially expressed lncRNA targets, circRNA cognate genes, and mRNAs were closely linked to many processes involved in spermatogenesis. In addition, some lncRNAs/circRNAs were greatly upregulated or inducibly expressed, implying their potential value as candidate markers for triptolide-induced male reproductive toxicity.
Conclusion: This study provides a preliminary database of triptolide-induced transcriptome, promotes understanding of the reproductive toxicity of triptolide, and highlights the need for research on increasing the medical efficacy of triptolide and decreasing its toxicity.
Keywords: RNA sequencing; Triptolide; circRNA; lncRNA; male infertility; spermatogenesis..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

