Osteoporosis: Current and Emerging Therapies Targeted to Immunological Checkpoints
- PMID: 31362684
- PMCID: PMC8206194
- DOI: 10.2174/0929867326666190730113123
Osteoporosis: Current and Emerging Therapies Targeted to Immunological Checkpoints
Abstract
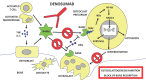
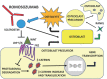
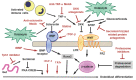
Osteoporosis is a skeletal pathology characterized by compromised bone strength leading to increased risk of fracture, mainly the spine and hip fractures. Osteoporosis affects more than 200 million people worldwide and because of the skeletal fractures it causes, represents a major cause of morbidity, disability and mortality in older people. Recently, the new discoveries of osteoimmunology have clarified many of the pathogenetic mechanisms of osteoporosis, helping to identify new immunological targets for its treatment opening the way for new and effective therapies with biological drugs. Currently, there are basically two monoclonal antibodies for osteoporosis therapy: denosumab and romosozumab. Here, we focus on the modern approach to the osteoporosis management and in particular, on current and developing biologic drugs targeted to new immunological checkpoints, in the landscape of osteoimmunology.
Keywords: Osteoporosis; biological therapies; bone remodeling; cytokines; immunological checkpoints; osteoimmunology.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures
References
-
- Nuti R., Brandi M.L., Checchia G., Di Munno O., Dominguez L., Falaschi P., Fiore C.E., Iolascon G., Maggi S., Michieli R., Migliaccio S., Minisola S., Rossini M., Sessa G., Tarantino U., Toselli A., Isaia G.C. Guidelines for the management of osteoporosis and fragility fractures. Intern. Emerg. Med. 2019;14(1):85–102. doi: 10.1007/s11739-018-1874-2. - DOI - PMC - PubMed
-
- Hernlund E., Svedbom A., Ivergård M., Compston J., Cooper C., Stenmark J., McCloskey E.V., Jönsson B., Kanis J.A. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013;8:136. doi: 10.1007/s11657-013-0136-1. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

