Mepolizumab reduces exacerbations in patients with severe eosinophilic asthma, irrespective of body weight/body mass index: meta-analysis of MENSA and MUSCA
- PMID: 31362741
- PMCID: PMC6664536
- DOI: 10.1186/s12931-019-1134-7
Mepolizumab reduces exacerbations in patients with severe eosinophilic asthma, irrespective of body weight/body mass index: meta-analysis of MENSA and MUSCA
Abstract
Background: We assessed the efficacy of the licensed mepolizumab dose (100 mg subcutaneously [SC]) in patients with severe eosinophilic asthma according to body weight/body mass index (BMI).
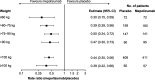
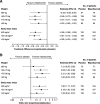
Methods: This was a post hoc individual patient-level meta-analysis of data from the Phase 3 studies MENSA (MEA115588/NCT01691521) and MUSCA (200862/NCT02281318). Patients aged ≥12 years with severe eosinophilic asthma and a history of exacerbations were randomised to 4-weekly placebo, mepolizumab 75 mg intravenously (IV) or 100 mg SC (MENSA) or placebo or mepolizumab 100 mg SC (MUSCA) for 32 (MENSA) or 24 (MUSCA) weeks. The primary endpoint was the annual rate of clinically significant exacerbations; other outcomes included the proportion of patients with no exacerbations, lung function, St George's Respiratory Questionnaire (SGRQ) and Asthma Control Questionnaire-5 (ACQ-5) scores and blood eosinophil counts. Analyses were performed by baseline body weight and BMI (≤60, > 60-75, > 75-90, > 90, < 100, ≥100 kg; ≤25, > 25-30, > 30, < 36, ≥36 kg/m2).
Results: Overall, 936 patients received placebo or mepolizumab 100 mg SC. Across all body weight/BMI categories, mepolizumab reduced the rate of clinically significant exacerbations by 49-70% versus placebo. Improvements with mepolizumab versus placebo were also seen in lung function in all body weight/BMI categories except > 90 kg; improvements in SGRQ and ACQ-5 scores were seen across all categories.
Conclusions: Mepolizumab 100 mg SC has consistent clinical benefits in patients with severe eosinophilic asthma across a range of body weights and BMIs. Data show that the fixed-dose regimen of mepolizumab is suitable, without the need for weight-based dosing.
Trial registration: This manuscript is a post hoc meta-analysis of data from the Phase 3 studies MENSA and MUSCA. ClinicalTrials.gov, NCT01691521 (MEA115588; MENSA). Registered September 24, 2012. ClinicalTrials.gov, NCT02281318 (200862; MUSCA). Registered November 3, 2014.
Keywords: Asthma; Asthma pharmacology; Body mass index; Body weight; Mepolizumab.
Conflict of interest statement
DJB, ESB, SWY and NK are employees of GSK and hold stocks/shares in GSK. FCA was an employee of GSK at the time of this analysis and holds stocks/shares in GSK. AP has received grants, personal fees and non-financial support and other from AstraZeneca, Teva, Mundipharma, GSK, Chiesi and Boehringer Ingelheim; has received personal fees and non-financial support from Novartis, Menarini and Zambon; and has received grants from Sanofi. CT has received fees from GSK, AstraZeneca, Novartis, Teva, Sanofi, Genzyme and Roche (consultancy, advisory boards) and was an investigator in trials involving GSK, AstraZeneca, Novartis, Sanofi, Roche and Boehringer Ingelheim.
Figures





References
-
- Global asthma report. http://globalasthmareport.org/. Accessed 16 Oct 2018.
-
- Mepolizumab (NUCALA) US prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125526Orig1s00.... Accessed 4 Dec 2018.
-
- Mepolizumab (NUCALA) EU summary of product characteristics. https://www.ema.europa.eu/documents/product-information/nucala-epar-prod.... Accessed 4 Dec 2018.

