Caffeine is associated with improved alveolarization and angiogenesis in male mice following hyperoxia induced lung injury
- PMID: 31362742
- PMCID: PMC6668145
- DOI: 10.1186/s12890-019-0903-x
Caffeine is associated with improved alveolarization and angiogenesis in male mice following hyperoxia induced lung injury
Abstract
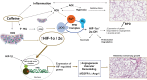
Background: Caffeine therapy for apnea of prematurity reduces the incidence of bronchopulmonary dysplasia (BPD) in premature neonates. Several mechanisms, including improvement in pulmonary mechanics underly beneficial effects of caffeine in BPD. As vascular development promotes alveologenesis, we hypothesized that caffeine might enhance angiogenesis in the lung, promoting lung growth, thereby attenuating BPD.
Methods: C57Bl/6 mice litters were randomized within 12 h of birth to room air (RA) or 95%O2 to receive caffeine (20 mg/kg/day) or placebo for 4 days and recovered in RA for 12wks. The lung mRNA and protein expression for hypoxia-inducible factors (HIF) and angiogenic genes performed on day 5. Lung morphometry and vascular remodeling assessed on inflation fixed lungs at 12wks.
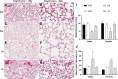
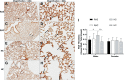
Results: Caffeine and hyperoxia in itself upregulate HIF-2α and vascular endothelial growth factor gene expression. Protein expression of HIF-2α and VEGFR1 were higher in hyperoxia/caffeine and angiopoietin-1 lower in hyperoxia. An increase in radial alveolar count, secondary septal count, and septal length with a decrease in mean linear intercept indicate an amelioration of hyperoxic lung injury by caffeine. An increase in vessel surface area and a significant reduction in smooth muscle thickness of the pulmonary arterioles may suggest a beneficial effect of caffeine on vascular remodeling in hyperoxia, especially in male mice.
Conclusions: Postnatal caffeine by modulating angiogenic gene expression early in lung development may restore the pulmonary microvasculature and alveolarization in adult lung.
Keywords: Caffeine; Hyperoxia; Hypoxia-inducible factors; Lung; Mice; Newborn; VEGF.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Anderson PJ, Doyle LW. Neurodevelopmental outcome of bronchopulmonary dysplasia. Semin Perinatol. 2006;30(4):227–232. - PubMed
-
- Greenough A. Long-term pulmonary outcome in the preterm infant. Neonatology. 2008;93(4):324–327. - PubMed
-
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354(20):2112–2121. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

