A detrimental role of RelB in mature oligodendrocytes during experimental acute encephalomyelitis
- PMID: 31362762
- PMCID: PMC6664766
- DOI: 10.1186/s12974-019-1548-7
A detrimental role of RelB in mature oligodendrocytes during experimental acute encephalomyelitis
Abstract
Background: Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system (CNS). It is firmly established that overactivation of the p65 (RelA) nuclear factor kappa B (NF-κB) transcription factor upregulates expression of inflammatory mediators in both immune and non-immune resident CNS cells and promotes inflammation during MS. In contrast to p65, NF-κB family member RelB regulates immune cell development and can limit inflammation. Although RelB expression is induced during inflammation in the CNS, its role in MS remains unknown.
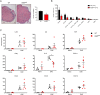
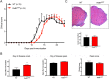
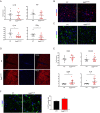
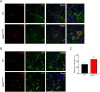
Methods: To examine the role of RelB in non-immune CNS cells, we generated mice with RelB specifically deleted in astrocytes (RelBΔAST), oligodendrocytes (RelBΔOLIGO), or neural progenitor-derived cells (RelBΔNP). We used experimental autoimmune encephalomyelitis (EAE), an accepted mouse model of MS, to assess the effect of RelB deletion on disease outcomes and performed analysis on the histological, cellular, and molecular level.
Results: Despite being a negative regulator of inflammation, conditional knockout of RelB in non-immune resident CNS cells surprisingly decreased the severity of EAE. This protective effect was recapitulated by conditional deletion of RelB in oligodendrocytes but not astrocytes. Deletion of RelB in oligodendrocytes reduced disease severity, promoted survival of mature oligodendrocytes, and correlated with increased activation of p65 NF-κB.
Conclusions: These findings suggest that RelB fine tunes inflammation and cell death/survival during EAE. Importantly, our data points out the detrimental role RelB plays in controlling survival of mature oligodendrocytes, which could be explored as a viable option to treat MS in the future.
Keywords: Astrocytes; EAE; Inflammation; NF-κB; Oligodendrocytes; RelB.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- McQualter JL, Bernard CC. Multiple sclerosis: a battle between destruction and repair. J Neurochem. 2007;100(2):295–306. - PubMed
-
- Lucchinetti C, Bruck W, Noseworthy J. Multiple sclerosis: recent developments in neuropathology, pathogenesis, magnetic resonance imaging studies and treatment. Curr Opin Neurol. 2001;14(3):259–269. - PubMed
-
- Hoffmann A, Natoli G, Ghosh G. Transcriptional regulation via the NF-kappaB signaling module. Oncogene. 2006;25(51):6706–6716. - PubMed
-
- Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3(3):221–227. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

