Development of a human immuno-oncology therapeutic agent targeting HER2: targeted delivery of granzyme B
- PMID: 31362764
- PMCID: PMC6668111
- DOI: 10.1186/s13046-019-1333-6
Development of a human immuno-oncology therapeutic agent targeting HER2: targeted delivery of granzyme B
Abstract
Background: Immunotherapeutic approaches designed to augment T and B cell mediated killing of tumor cells has met with clinical success in recent years suggesting tremendous potential for treatment in a broad spectrum of tumor types. After complex recognition of target cells by T and B cells, delivery of the serine protease granzyme B (GrB) to tumor cells comprises the cytotoxic insult resulting in a well-characterized, multimodal apoptotic cascade.
Methods: We designed a recombinant fusion construct, GrB-Fc-4D5, composed of a humanized anti-HER2 scFv fused to active GrB for recognition of tumor cells and internal delivery of GrB, simulating T and B cell therapy. We assessed the construct's antigen-binding specificity and GrB enzymatic activity, as well as in vitro cytotoxicity and internalization into target and control cells. We also assessed pharmacokinetic and toxicology parameters in vivo.
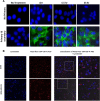
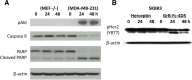
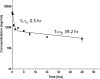
Results: GrB-Fc-4D5 was highly cytotoxic to Her2 positive cells such as SKBR3, MCF7 and MDA-MB-231 with IC50 values of 56, 99 and 27 nM, respectively, and against a panel of HER2+ cell lines regardless of endogenous expression levels of the PI-9 inhibitor. Contemporaneous studies with Kadcyla demonstrated similar levels of in vitro activity against virtually all cells tested. GrB-Fc-4D5 internalized rapidly into target SKOV3 cells within 1 h of exposure rapidly delivering GrB to the cytoplasmic compartment. In keeping with its relatively high molecular weight (160 kDa), the construct demonstrated a terminal-phase serum half-life in mice of 39.2 h. Toxicity studies conducted on BALB/c mice demonstrated no statistically significant changes in SGPT, SGOT or serum LDH. Histopathologic analysis of tissues from treated mice demonstrated no drug-related changes in any tissues examined.
Conclusion: GrB-Fc-4D5 shows excellent, specific cytotoxicity and demonstrates no significant toxicity in normal, antigen-negative murine models. This construct constitutes a novel approach against HER2-expressing tumors and is an excellent candidate for further development.
Keywords: Granzyme B; HER2; Immunotherapy; Kadcyla; Pharmacokinetics.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Duong CP, Yong CS, Kershaw MH, Slaney CY, Darcy PK. Cancer immunotherapy utilizing gene-modified T cells: From the bench to the clinic. Mol Immunol. 2015;67(2 Pt A):46–57. - PubMed
-
- Ekkirala CR, Cappello P, Accolla RS, Giovarelli M, Romero I, Garrido C, et al. Class II transactivator-induced MHC class II expression in pancreatic cancer cells leads to tumor rejection and a specific antitumor memory response. Pancreas. 2014;43(7):1066–1072. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

