Combination therapy targeting both innate and adaptive immunity improves survival in a pre-clinical model of ovarian cancer
- PMID: 31362778
- PMCID: PMC6668091
- DOI: 10.1186/s40425-019-0654-5
Combination therapy targeting both innate and adaptive immunity improves survival in a pre-clinical model of ovarian cancer
Abstract
Background: Despite major advancements in immunotherapy among a number of solid tumors, response rates among ovarian cancer patients remain modest. Standard treatment for ovarian cancer is still surgery followed by taxane- and platinum-based chemotherapy. Thus, there is an urgent need to develop novel treatment options for clinical translation.
Methods: Our approach was to analyze the effects of standard chemotherapy in the tumor microenvironment of mice harboring orthotopic, syngeneic ID8-Vegf-Defb29 ovarian tumors in order to mechanistically determine a complementary immunotherapy combination. Specifically, we interrogated the molecular and cellular consequences of chemotherapy by analyzing gene expression and flow cytometry data.
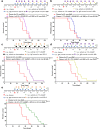
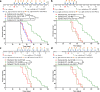
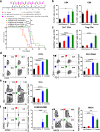
Results: These data show that there is an immunosuppressive shift in the myeloid compartment, with increased expression of IL-10 and ARG1, but no activation of CD3+ T cells shortly after chemotherapy treatment. We therefore selected immunotherapies that target both the innate and adaptive arms of the immune system. Survival studies revealed that standard chemotherapy was complemented most effectively by a combination of anti-IL-10, 2'3'-cGAMP, and anti-PD-L1. Immunotherapy dramatically decreased the immunosuppressive myeloid population while chemotherapy effectively activated dendritic cells. Together, combination treatment increased the number of activated T and dendritic cells as well as expression of cytotoxic factors. It was also determined that the immunotherapy had to be administered concurrently with the chemotherapy to reverse the acute immunosuppression caused by chemotherapy. Mechanistic studies revealed that antitumor immunity in this context was driven by CD4+ T cells, which acquired a highly activated phenotype. Our data suggest that these CD4+ T cells can kill cancer cells directly via granzyme B-mediated cytotoxicity. Finally, we showed that this combination therapy is also effective at delaying tumor growth substantially in an aggressive model of lung cancer, which is also treated clinically with taxane- and platinum-based chemotherapy.
Conclusions: This work highlights the importance of CD4+ T cells in tumor immunology. Furthermore, the data support the initiation of clinical trials in ovarian cancer that target both innate and adaptive immunity, with a focus on optimizing dosing schedules.
Keywords: CD4+ T cells; Cancer immunotherapy; Combination therapy; Innate immunity; Ovarian cancer.
Conflict of interest statement
J.L.G. is a consultant for GSK and receives sponsored research support from GSK and Eli Lilly. No other authors have any conflicts related to this work.
Figures



References
-
- ACS. Cancer Facts & Figures. Atlanta: American Cancer Society; 2018.
-
- ORCFA. Statistics: Ovarian Cancer Resarch Fund Alliance, New York, New York, USA; 2018
-
- Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
