Phase I and phase II sonidegib and vismodegib clinical trials for the treatment of paediatric and adult MB patients: a systemic review and meta-analysis
- PMID: 31362788
- PMCID: PMC6668073
- DOI: 10.1186/s40478-019-0773-8
Phase I and phase II sonidegib and vismodegib clinical trials for the treatment of paediatric and adult MB patients: a systemic review and meta-analysis
Abstract
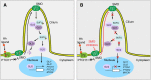
Background: Medulloblastoma (MB) is the most common malignant brain tumour in children but also rarely occur in adults. Sonic Hedgehog (SHH) driven MB is associated with aberrant activation of the SHH signalling pathway. SMO inhibitors, sonidegib and vismodegib, have been used as selective antagonist of the hedgehog pathway that acts by binding to SMO, and inhibits activation of the downstream hedgehog target genes. Several clinical trials investigating SMO inhibitors for the treatment of relapsed MB patients have been published.
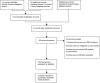
Methods: We conducted a systemic review and meta-analysis among these Phase I and II clinical trials. The pooled effect of SMO inhibitors in relapsed MB were analysed using Reviewer Manager 5.3 software. The clinical efficacy of SMO inhibitors on SHH subtype of MB were measured by the objective response rate. The risk difference was obtained by comparing the ORR between SHH and non-SHH subtypes of MB.
Results: The five studies all had clear criteria for patient recruitment, adequate follow-up time for endpoint assessment and clear definition of tumour responses. MB patients had good compliance in the trials. The pooled objective response rate (ORR) of SMO inhibitor was 37% and 0 against SHH-driven and other MBs. The pooled ORR of sonidegib was 55% among MBSHH and 0 among MBnon-SHH subgroup. Vismodegib also had no efficacy on non-SHH subtype of MB. The sonidegib against SHH-driven MB produced the ORR 1.87-fold higher than that of vismodegib (95%CI 1.23, 6.69). Among paediatric patients, the efficacy of sonidegib was 3.67-fold higher than vismodegib (p < 0.05). A total of 320 cases received SMO inhibitor therapy and 36 cases reported grade 3/4 dose-limiting toxicity (DLT). The rate of grade 3/4 DLT was similar between patients receiving vismodegib and sonidegib (11.6% vs. 11.2%).
Conclusion: Sonidegib and vismodegib were well tolerated and demonstrated anti-tumour activity in SHH-driven paediatric and adult MB by effectively inhibiting Hh signalling. These results support the ongoing clinical trials using SMO inhibitors in combination with conventional chemotherapies for the treatment of relapsed MBSHH.
Keywords: And vismodegib; Medulloblastoma; SMO inhibitor; Sonic hedgehog pathway; Sonidegib.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

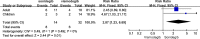
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

