Effectiveness of First-line Immune Checkpoint Blockade Versus Carboplatin-based Chemotherapy for Metastatic Urothelial Cancer
- PMID: 31362898
- PMCID: PMC6822167
- DOI: 10.1016/j.eururo.2019.07.032
Effectiveness of First-line Immune Checkpoint Blockade Versus Carboplatin-based Chemotherapy for Metastatic Urothelial Cancer
Abstract
Background: Limited data compare first-line carboplatin-based chemotherapy and immune checkpoint blockade in cisplatin-ineligible metastatic urothelial carcinoma (mUC) patients. The primary evidence guiding treatment decisions was a recent Food and Drug Administration/European Medicines Agency safety alert based on emerging data from two ongoing phase III trials, reporting shorter survival in programmed death-ligand 1 (PD-L1)-negative patients receiving immunotherapy. Final results from these trials are unknown.
Objective: To compare survival in cisplatin-ineligible mUC patients receiving first-line immunotherapy versus those receiving carboplatin-based chemotherapy.
Design, setting, and participants: We conducted a retrospective cohort study of 2017 mUC patients receiving first-line carboplatin-based chemotherapy (n = 1530) or immunotherapy (n = 487) from January 1, 2011 to May 18, 2018 using the Flatiron Health electronic health record-derived database.
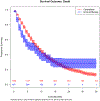
Outcome measurements and statistical analysis: The primary outcomes were overall survival (OS), comparing 12- and 36-mo OS, and hazard ratios before and after 12 mo. Propensity score-based inverse probability of treatment weighting (IPTW) was used to address confounding in Kaplan-Meier and Cox regression model estimates of comparative effectiveness.
Results and limitations: IPTW-adjusted OS rates in the immunotherapy group were lower at 12 mo (39.6% [95% confidence interval {CI} 34.0-45.3%] vs 46.1% [95% CI 43.4-48.8%]) but higher at 36 mo (28.3% [95% CI 21.8-34.7%] vs 13.3% [95% CI 11.1-15.5%]) relative to the chemotherapy group. Immunotherapy treatment demonstrated inferior OS during the first 12 mo relative to carboplatin-based chemotherapy (IPTW-adjusted hazard ratio [HR] 1.37, 95% CI 1.15-1.62), but superior OS beyond 12 mo (IPTW-adjusted HR 0.50, 95% CI 0.30-0.85). Limitations include retrospective design and potential unmeasured confounding.
Conclusions: In the setting of mUC, clinicians and patients should carefully consider how to balance the short-term benefit of chemotherapy against the long-term benefit of immunotherapy.
Patient summary: To determine the optimal first-line therapy for metastatic bladder cancer patients who are unfit for cisplatin, we compared carboplatin-based chemotherapy versus immunotherapy using real-world data. Survival in the 1st year of treatment was lower with immunotherapy relative to chemotherapy, but for patients surviving beyond the 1st year, immunotherapy was superior.
Keywords: European Medicines Agency; Food and Drug Administration; Immune checkpoint blockade; Metastatic urothelial cancer; Real-world data.
Copyright © 2019 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Figures
Comment in
-
Optimizing frontline therapy in advanced urothelial cancer.Transl Androl Urol. 2020 Jun;9(3):983-985. doi: 10.21037/tau.2020.04.03. Transl Androl Urol. 2020. PMID: 32676379 Free PMC article. No abstract available.
-
First-line immune checkpoint inhibitors for patients with metastatic urothelial carcinoma treated in routine clinical practice.Transl Androl Urol. 2020 Jun;9(3):986-990. doi: 10.21037/tau.2020.04.08. Transl Androl Urol. 2020. PMID: 32676380 Free PMC article. No abstract available.
References
-
- von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. Journal of Clinical Oncology. 2000;18(17):3068–3077. - PubMed
-
- Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. Journal of Clinical Oncology. 2011;29(17):2432–2438. - PubMed
-
- De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. Journal of Clinical Oncology. 2012;30(2):191–199. - PMC - PubMed
-
- Necchi A, Pond GR, Raggi D, et al. Efficacy and safety of gemcitabine plus either taxane or carboplatin in the first-line setting of metastatic urothelial carcinoma: A systematic review and meta-analysis. Clinical Genitourinary Cancer. 2017;15(1):23–30. - PubMed
-
- Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. The Lancet Oncology. 2017;18(11):1483–1492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials