Interplay between HGAL and Grb2 proteins regulates B-cell receptor signaling
- PMID: 31362927
- PMCID: PMC6693015
- DOI: 10.1182/bloodadvances.2018016162
Interplay between HGAL and Grb2 proteins regulates B-cell receptor signaling
Abstract
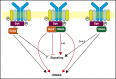
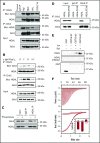
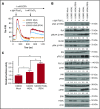
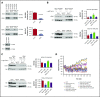
Human germinal center (GC)-associated lymphoma (HGAL) is an adaptor protein expressed in GC B cells. HGAL regulates cell motility and B-cell receptor (BCR) signaling, processes that are central for the successful completion of the GC reaction. Herein, we demonstrate phosphorylation of HGAL by Syk and Lyn kinases at tyrosines Y80, Y86, Y106Y107, Y128, and Y148. The HGAL YEN motif (amino acids 107-109) is similar to the phosphopeptide motif pYXN used as a binding site to the growth factor receptor-bound protein 2 (Grb2). We demonstrate by biochemical and molecular methodologies that HGAL directly interacts with Grb2. Concordantly, microscopy studies demonstrate HGAL-Grb2 colocalization in the membrane central supramolecular activation clusters (cSMAC) following BCR activation. Mutation of the HGAL putative binding site to Grb2 abrogates the interaction between these proteins. Further, this HGAL mutant localizes exclusively in the peripheral SMAC and decreases the rate and intensity of BCR accumulation in the cSMAC. Furthermore, we demonstrate that Grb2, HGAL, and Syk interact in the same complex, but Grb2 does not modulate the effects of HGAL on Syk kinase activity. Overall, the interplay between the HGAL and Grb2 regulates the magnitude of BCR signaling and synapse formation.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


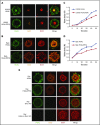
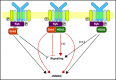
Similar articles
-
HGAL localization to cell membrane regulates B-cell receptor signaling.Blood. 2015 Jan 22;125(4):649-57. doi: 10.1182/blood-2014-04-571331. Epub 2014 Nov 7. Blood. 2015. PMID: 25381061 Free PMC article.
-
Regulation of BCR-dependent germinal center B-cell formation by HGAL and insight into its emerging myeloid ortholog, C1ORF150.Front Immunol. 2024 Oct 15;15:1437516. doi: 10.3389/fimmu.2024.1437516. eCollection 2024. Front Immunol. 2024. PMID: 39474423 Free PMC article. Review.
-
Studies of a germinal centre B-cell expressed gene, GCET2, suggest its role as a membrane associated adapter protein.Br J Haematol. 2007 Jun;137(6):578-90. doi: 10.1111/j.1365-2141.2007.06597.x. Epub 2007 May 9. Br J Haematol. 2007. PMID: 17489982 Free PMC article.
-
The Dok-3/Grb2 protein signal module attenuates Lyn kinase-dependent activation of Syk kinase in B cell antigen receptor microclusters.J Biol Chem. 2013 Jan 25;288(4):2303-13. doi: 10.1074/jbc.M112.406546. Epub 2012 Dec 5. J Biol Chem. 2013. PMID: 23223229 Free PMC article.
-
Structural aspects of signal transduction in B-cells.Crit Rev Immunol. 1996;16(3):251-75. doi: 10.1615/critrevimmunol.v16.i3.20. Crit Rev Immunol. 1996. PMID: 8922899 Review.
Cited by
-
FCRL1 Regulates B Cell Receptor-Induced ERK Activation through GRB2.J Immunol. 2021 Dec 1;207(11):2688-2698. doi: 10.4049/jimmunol.2100218. Epub 2021 Oct 25. J Immunol. 2021. PMID: 34697226 Free PMC article.
-
Detection of new drivers of frequent B-cell lymphoid neoplasms using an integrated analysis of whole genomes.PLoS One. 2021 May 4;16(5):e0248886. doi: 10.1371/journal.pone.0248886. eCollection 2021. PLoS One. 2021. PMID: 33945543 Free PMC article.
-
Whole-Transcriptome Profiling and circRNA-miRNA-mRNA Regulatory Networks in B-Cell Development.Front Immunol. 2022 Mar 21;13:812924. doi: 10.3389/fimmu.2022.812924. eCollection 2022. Front Immunol. 2022. PMID: 35386709 Free PMC article.
-
HGAL inhibits lymphoma dissemination by interacting with multiple cytoskeletal proteins.Blood Adv. 2021 Dec 14;5(23):5072-5085. doi: 10.1182/bloodadvances.2021004304. Blood Adv. 2021. PMID: 34543391 Free PMC article.
-
Conditional expression of HGAL leads to the development of diffuse large B-cell lymphoma in mice.Blood. 2021 Apr 1;137(13):1741-1753. doi: 10.1182/blood.2020004996. Blood. 2021. PMID: 33024996 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

