Deletion of the transmembrane protein Prom1b in zebrafish disrupts outer-segment morphogenesis and causes photoreceptor degeneration
- PMID: 31362982
- PMCID: PMC6755801
- DOI: 10.1074/jbc.RA119.008618
Deletion of the transmembrane protein Prom1b in zebrafish disrupts outer-segment morphogenesis and causes photoreceptor degeneration
Abstract
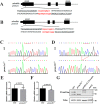
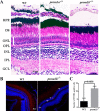
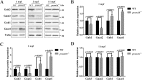
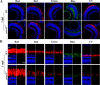
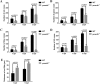
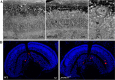
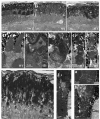
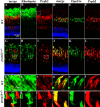
Mutations in human prominin 1 (PROM1), encoding a transmembrane glycoprotein localized mainly to plasma membrane protrusions, have been reported to cause retinitis pigmentosa, macular degeneration, and cone-rod dystrophy. Although the structural role of PROM1 in outer-segment (OS) morphogenesis has been demonstrated in Prom1-knockout mouse, the mechanisms underlying these complex disease phenotypes remain unclear. Here, we utilized a zebrafish model to further investigate PROM1's role in the retina. The Prom1 orthologs in zebrafish include prom1a and prom1b, and our results showed that prom1b, rather than prom1a, plays an important role in zebrafish photoreceptors. Loss of prom1b disrupted OS morphogenesis, with rods and cones exhibiting differences in impairment: cones degenerated at an early age, whereas rods remained viable but with an abnormal OS, even at 9 months postfertilization. Immunofluorescence experiments with WT zebrafish revealed that Prph2, an ortholog of the human transmembrane protein peripherin 2 and also associated with OS formation, is localized to the edge of OS and is more highly expressed in the cone OS than in the rod OS. Moreover, we found that Prom1b deletion causes mislocalization of Prph2 and disrupts its oligomerization. We conclude that the variation in Prph2 levels between cones and rods was one of the reasons for the different PROM1 mutation-induced phenotypes of these retinal structures. These findings expand our understanding of the phenotypes caused by PROM1 mutations and provide critical insights into its function.
Keywords: Danio rerio; development; eye disease; morphogenesis; peripherin 2 (PRPH2); photoreceptor; prominin 1 (PROM1); retinal degeneration; zebrafish.
© 2019 Lu et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
Comment in
-
Deciphering the roles of prominins in the visual system.J Biol Chem. 2019 Nov 8;294(45):17166. doi: 10.1074/jbc.L119.011198. J Biol Chem. 2019. PMID: 31704774 Free PMC article. No abstract available.
-
Reply to Corbeil et al.: Deletion of the transmembrane protein Prom1b in zebrafish disrupts outer-segment morphogenesis and causes photoreceptor degeneration.J Biol Chem. 2019 Nov 8;294(45):17167. doi: 10.1074/jbc.RL119.011224. J Biol Chem. 2019. PMID: 31704775 Free PMC article. No abstract available.
References
-
- Zacchigna S., Oh H., Wilsch-Bräuninger M., Missol-Kolka E., Jászai J., Jansen S., Tanimoto N., Tonagel F., Seeliger M., Huttner W. B., Corbeil D., Dewerchin M., Vinckier S., Moons L., and Carmeliet P. (2009) Loss of the cholesterol-binding protein prominin-1/CD133 causes disk dysmorphogenesis and photoreceptor degeneration. J. Neurosci. 29, 2297–2308 10.1523/JNEUROSCI.2034-08.2009 - DOI - PMC - PubMed
-
- Miraglia S., Godfrey W., Yin A. H., Atkins K., Warnke R., Holden J. T., Bray R. A., Waller E. K., and Buck D. W. (1997) A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood 90, 5013–5021 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

