Xenogeneic modulation of the ClpCP protease of Bacillus subtilis by a phage-encoded adaptor-like protein
- PMID: 31362989
- PMCID: PMC6873191
- DOI: 10.1074/jbc.RA119.010007
Xenogeneic modulation of the ClpCP protease of Bacillus subtilis by a phage-encoded adaptor-like protein
Abstract
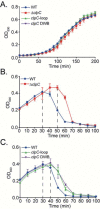
Like eukaryotic and archaeal viruses, which coopt the host's cellular pathways for their replication, bacteriophages have evolved strategies to alter the metabolism of their bacterial host. SPO1 bacteriophage infection of Bacillus subtilis results in comprehensive remodeling of cellular processes, leading to conversion of the bacterial cell into a factory for phage progeny production. A cluster of 26 genes in the SPO1 genome, called the host takeover module, encodes for potentially cytotoxic proteins that specifically shut down various processes in the bacterial host, including transcription, DNA synthesis, and cell division. However, the properties and bacterial targets of many genes of the SPO1 host takeover module remain elusive. Through a systematic analysis of gene products encoded by the SPO1 host takeover module, here we identified eight gene products that attenuated B. subtilis growth. Of the eight phage gene products that attenuated bacterial growth, a 25-kDa protein called Gp53 was shown to interact with the AAA+ chaperone protein ClpC of the ClpCP protease of B. subtilis Our results further reveal that Gp53 is a phage-encoded adaptor-like protein that modulates the activity of the ClpCP protease to enable efficient SPO1 phage progeny development. In summary, our findings indicate that the bacterial ClpCP protease is the target of xenogeneic (dys)regulation by a SPO1 phage-derived factor and add Gp53 to the list of antibacterial products that target bacterial protein degradation and therefore may have utility for the development of novel antibacterial agents.
Keywords: ATP-dependent protease; Bacillus; bacteria; bacteriophage; chaperone.
© 2019 Mulvenna et al.
Figures



Similar articles
-
The product of SPO1 gene 56 inhibits host cell division during infection of Bacillus subtilis by bacteriophage SPO1.Virology. 2013 Dec;447(1-2):249-53. doi: 10.1016/j.virol.2013.09.005. Epub 2013 Oct 6. Virology. 2013. PMID: 24210121
-
Analysis of Host-Takeover During SPO1 Infection of Bacillus subtilis.Methods Mol Biol. 2018;1681:31-39. doi: 10.1007/978-1-4939-7343-9_2. Methods Mol Biol. 2018. PMID: 29134584
-
Phage SPO1 Protein Gp49 Is a Novel RNA Binding Protein That Is Involved in Host Iron Metabolism.Int J Mol Sci. 2023 Sep 20;24(18):14318. doi: 10.3390/ijms241814318. Int J Mol Sci. 2023. PMID: 37762620 Free PMC article.
-
Role of host factors in bacteriophage φ29 DNA replication.Adv Virus Res. 2012;82:351-83. doi: 10.1016/B978-0-12-394621-8.00020-0. Adv Virus Res. 2012. PMID: 22420858 Review.
-
The SPO1-related bacteriophages.Arch Virol. 2010 Oct;155(10):1547-61. doi: 10.1007/s00705-010-0783-0. Epub 2010 Aug 17. Arch Virol. 2010. PMID: 20714761 Review.
Cited by
-
PrkA controls peptidoglycan biosynthesis through the essential phosphorylation of ReoM.Elife. 2020 May 29;9:e56048. doi: 10.7554/eLife.56048. Elife. 2020. PMID: 32469310 Free PMC article.
-
A tunable anthranilate-inducible gene expression system for Pseudomonas species.Appl Microbiol Biotechnol. 2021 Jan;105(1):247-258. doi: 10.1007/s00253-020-11034-8. Epub 2020 Dec 3. Appl Microbiol Biotechnol. 2021. PMID: 33270152 Free PMC article.
-
Bacteriophage protein Gp46 is a cross-species inhibitor of nucleoid-associated HU proteins.Proc Natl Acad Sci U S A. 2022 Mar 1;119(9):e2116278119. doi: 10.1073/pnas.2116278119. Proc Natl Acad Sci U S A. 2022. PMID: 35193978 Free PMC article.
-
Bacteriophages of Thermophilic 'Bacillus Group' Bacteria-A Review.Microorganisms. 2021 Jul 16;9(7):1522. doi: 10.3390/microorganisms9071522. Microorganisms. 2021. PMID: 34361957 Free PMC article. Review.
-
MecA: A Multifunctional ClpP-Dependent and Independent Regulator in Gram-Positive Bacteria.Mol Microbiol. 2025 May;123(5):433-438. doi: 10.1111/mmi.15356. Epub 2025 Mar 11. Mol Microbiol. 2025. PMID: 40070161 Review.
References
-
- Stewart C. R., Gaslightwala I., Hinata K., Krolikowski K. A., Needleman D. S., Peng A. S., Peterman M. A., Tobias A., and Wei P. (1998) Genes and regulatory sites of the “host-takeover module” in the terminal redundancy of Bacillus subtilis bacteriophage SPO1. Virology 246, 329–340 10.1006/viro.1998.9197 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

