Endogenous Retrovirus-Derived Long Noncoding RNA Enhances Innate Immune Responses via Derepressing RELA Expression
- PMID: 31363026
- PMCID: PMC6667616
- DOI: 10.1128/mBio.00937-19
Endogenous Retrovirus-Derived Long Noncoding RNA Enhances Innate Immune Responses via Derepressing RELA Expression
Abstract
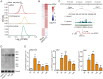
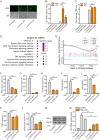
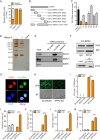
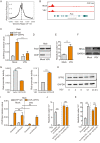
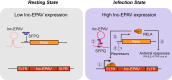
Endogenous retroviruses (ERVs) are transposable elements that cause host genome instability and usually play deleterious roles in disease such as tumorigenesis. Recent advances also suggest that this "enemy within" may encode a viral mimic to induce antiviral immune responses through viral sensors. Here, through whole-genome transcriptome analysis with RNA sequencing (RNA-Seq), we discovered that a full-length ERV-derived long noncoding RNA (lncRNA), designated lnc-EPAV (ERV-derived lncRNA positively regulates antiviral responses), was a positive regulator of NF-κB signaling. lnc-EPAV expression was rapidly upregulated by viral RNA mimics or RNA viruses to facilitate the expression of RELA, an NF-κB subunit that plays a crucial role in antiviral responses. Transcriptome analysis of lnc-EPAV-silenced macrophages showed that lnc-EPAV was critical for RELA target gene expression and innate immune responses. Consistently, lnc-EPAV-deficient mice exhibited reduced expression of type I interferons (IFNs) and, consequently, increased viral loads and mortality following lethal RNA virus infection. Mechanistically, lnc-EPAV promoted expression of RELA by competitively binding to and displacing SFPQ, a transcriptional repressor of Rela Altogether, our work demonstrates an alternative mechanism by which ERVs regulate antiviral immune responses.IMPORTANCE Endogenous retroviruses are transposable genetic elements comprising 8% to 10% of the human and mouse genomes. Although most ERVs have been inactivated due to deleterious mutations, some are still transcribed. However, the biological functions of transcribed ERVs are largely unknown. Here, we identified a full-length ERV-derived lncRNA, designated lnc-EPAV, as a positive regulator of host innate immune responses. We found that silencing lnc-EPAV impaired virus-induced cytokine production, resulting in increased viral replication in cells. The lnc-EPAV-deficient mice exhibited enhanced susceptibility to viral challenge. We also found that lnc-EPAV regulated expression of RELA, an NF-κB subunit that plays a critical role in antiviral responses. ERV-derived lncRNA coordinated with a transcription repressor, SFPQ, to control Rela transcription. Our report provides new insights into the previously unrecognized immune gene regulatory mechanism of ERV-derived lncRNAs.
Keywords: NF-κB; antiviral immune responses; endogenous retroviruses; gene regulation; lncRNA.
Copyright © 2019 Zhou et al.
Figures

Similar articles
-
Long Noncoding RNA Lnc-MxA Inhibits Beta Interferon Transcription by Forming RNA-DNA Triplexes at Its Promoter.J Virol. 2019 Oct 15;93(21):e00786-19. doi: 10.1128/JVI.00786-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31434735 Free PMC article.
-
The Long Noncoding RNA NEAT1 Exerts Antihantaviral Effects by Acting as Positive Feedback for RIG-I Signaling.J Virol. 2017 Apr 13;91(9):e02250-16. doi: 10.1128/JVI.02250-16. Print 2017 May 1. J Virol. 2017. PMID: 28202761 Free PMC article.
-
An endogenous retroviral element exerts an antiviral innate immune function via the derived lncRNA lnc-ALVE1-AS1.Antiviral Res. 2019 Oct;170:104571. doi: 10.1016/j.antiviral.2019.104571. Epub 2019 Jul 30. Antiviral Res. 2019. PMID: 31374219
-
The Opening of Pandora's Box: An Emerging Role of Long Noncoding RNA in Viral Infections.Front Immunol. 2019 Jan 25;9:3138. doi: 10.3389/fimmu.2018.03138. eCollection 2018. Front Immunol. 2019. PMID: 30740112 Free PMC article. Review.
-
The potential roles of endogenous retroviruses in autoimmunity.Immunol Rev. 1996 Aug;152:193-236. doi: 10.1111/j.1600-065x.1996.tb00917.x. Immunol Rev. 1996. PMID: 8930674 Review.
Cited by
-
Functional Bidirectionality of ERV-Derived Long Non-Coding RNAs in Humans.Int J Mol Sci. 2024 Sep 29;25(19):10481. doi: 10.3390/ijms251910481. Int J Mol Sci. 2024. PMID: 39408810 Free PMC article. Review.
-
COVID-19: The Ethno-Geographic Perspective of Differential Immunity.Vaccines (Basel). 2023 Jan 31;11(2):319. doi: 10.3390/vaccines11020319. Vaccines (Basel). 2023. PMID: 36851197 Free PMC article. Review.
-
Genome-Wide Characterization of Zebrafish Endogenous Retroviruses Reveals Unexpected Diversity in Genetic Organizations and Functional Potentials.Microbiol Spectr. 2021 Dec 22;9(3):e0225421. doi: 10.1128/spectrum.02254-21. Epub 2021 Dec 15. Microbiol Spectr. 2021. PMID: 34908463 Free PMC article.
-
From genotype to phenotype: genetics of mammalian long non-coding RNAs in vivo.Nat Rev Genet. 2022 Apr;23(4):229-243. doi: 10.1038/s41576-021-00427-8. Epub 2021 Nov 26. Nat Rev Genet. 2022. PMID: 34837040 Review.
-
Transcriptional Regulation of Endogenous Retroviruses and Their Misregulation in Human Diseases.Int J Mol Sci. 2022 Sep 4;23(17):10112. doi: 10.3390/ijms231710112. Int J Mol Sci. 2022. PMID: 36077510 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

