Regulation of Glutarate Catabolism by GntR Family Regulator CsiR and LysR Family Regulator GcdR in Pseudomonas putida KT2440
- PMID: 31363033
- PMCID: PMC6667623
- DOI: 10.1128/mBio.01570-19
Regulation of Glutarate Catabolism by GntR Family Regulator CsiR and LysR Family Regulator GcdR in Pseudomonas putida KT2440
Abstract
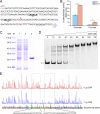
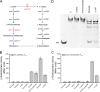
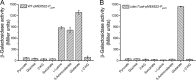
Glutarate, a metabolic intermediate in the catabolism of several amino acids and aromatic compounds, can be catabolized through both the glutarate hydroxylation pathway and the glutaryl-coenzyme A (glutaryl-CoA) dehydrogenation pathway in Pseudomonas putida KT2440. The elucidation of the regulatory mechanism could greatly aid in the design of biotechnological alternatives for glutarate production. In this study, it was found that a GntR family protein, CsiR, and a LysR family protein, GcdR, regulate the catabolism of glutarate by repressing the transcription of csiD and lhgO, two key genes in the glutarate hydroxylation pathway, and by activating the transcription of gcdH and gcoT, two key genes in the glutaryl-CoA dehydrogenation pathway, respectively. Our data suggest that CsiR and GcdR are independent and that there is no cross-regulation between the two pathways. l-2-Hydroxyglutarate (l-2-HG), a metabolic intermediate in the glutarate catabolism with various physiological functions, has never been elucidated in terms of its metabolic regulation. Here, we reveal that two molecules, glutarate and l-2-HG, act as effectors of CsiR and that P. putida KT2440 uses CsiR to sense glutarate and l-2-HG and to utilize them effectively. This report broadens our understanding of the bacterial regulatory mechanisms of glutarate and l-2-HG catabolism and may help to identify regulators of l-2-HG catabolism in other species.IMPORTANCE Glutarate is an attractive dicarboxylate with various applications. Clarification of the regulatory mechanism of glutarate catabolism could help to block the glutarate catabolic pathways, thereby improving glutarate production through biotechnological routes. Glutarate is a toxic metabolite in humans, and its accumulation leads to a hereditary metabolic disorder, glutaric aciduria type I. The elucidation of the functions of CsiR and GcdR as regulators that respond to glutarate could help in the design of glutarate biosensors for the rapid detection of glutarate in patients with glutaric aciduria type I. In addition, CsiR was identified as a regulator that also regulates l-2-HG metabolism. The identification of CsiR as a regulator that responds to l-2-HG could help in the discovery and investigation of other regulatory proteins involved in l-2-HG catabolism.
Keywords: catabolism; glutarate; l-2-hydroxyglutarate; regulatory mechanism.
Copyright © 2019 Zhang et al.
Figures





Similar articles
-
Increased glutarate production by blocking the glutaryl-CoA dehydrogenation pathway and a catabolic pathway involving L-2-hydroxyglutarate.Nat Commun. 2018 May 29;9(1):2114. doi: 10.1038/s41467-018-04513-0. Nat Commun. 2018. PMID: 29844506 Free PMC article.
-
Robust Characterization of Two Distinct Glutarate Sensing Transcription Factors of Pseudomonas putida l-Lysine Metabolism.ACS Synth Biol. 2019 Oct 18;8(10):2385-2396. doi: 10.1021/acssynbio.9b00255. Epub 2019 Sep 25. ACS Synth Biol. 2019. PMID: 31518500
-
Molecular characterization of lysR-lysXE, gcdR-gcdHG and amaR-amaAB operons for lysine export and catabolism: a comprehensive lysine catabolic network in Pseudomonas aeruginosa PAO1.Microbiology (Reading). 2016 May;162(5):876-888. doi: 10.1099/mic.0.000277. Epub 2016 Mar 11. Microbiology (Reading). 2016. PMID: 26967762
-
Widespread bacterial lysine degradation proceeding via glutarate and L-2-hydroxyglutarate.Nat Commun. 2018 Nov 29;9(1):5071. doi: 10.1038/s41467-018-07563-6. Nat Commun. 2018. PMID: 30498244 Free PMC article.
-
Bacterial catabolism of nicotine: Catabolic strains, pathways and modules.Environ Res. 2020 Apr;183:109258. doi: 10.1016/j.envres.2020.109258. Epub 2020 Feb 19. Environ Res. 2020. PMID: 32311908 Review.
Cited by
-
Enhancing glutaric acid production in Escherichia coli by uptake of malonic acid.J Ind Microbiol Biotechnol. 2020 Mar;47(3):311-318. doi: 10.1007/s10295-020-02268-6. Epub 2020 Mar 5. J Ind Microbiol Biotechnol. 2020. PMID: 32140931
-
Dehydrogenation Mechanism of Three Stereoisomers of Butane-2,3-Diol in Pseudomonas putida KT2440.Front Bioeng Biotechnol. 2021 Aug 26;9:728767. doi: 10.3389/fbioe.2021.728767. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34513815 Free PMC article.
-
Genome analysis of Pseudomonas chlororaphis subsp. aurantiaca mutant strains with increased production of phenazines.Arch Microbiol. 2022 Apr 9;204(5):247. doi: 10.1007/s00203-021-02648-1. Arch Microbiol. 2022. PMID: 35397008
-
An Ultrasensitive Biosensor for Probing Subcellular Distribution and Mitochondrial Transport of l-2-Hydroxyglutarate.Adv Sci (Weinh). 2024 Sep;11(35):e2404119. doi: 10.1002/advs.202404119. Epub 2024 Jul 15. Adv Sci (Weinh). 2024. PMID: 39005231 Free PMC article.
-
A Novel Transcription Factor VPA0041 Was Identified to Regulate the Swarming Motility in Vibrio parahaemolyticus.Pathogens. 2022 Apr 10;11(4):453. doi: 10.3390/pathogens11040453. Pathogens. 2022. PMID: 35456128 Free PMC article.
References
-
- Kim HT, Khang TU, Baritugo KA, Hyun SM, Kang KH, Jung SH, Song BK, Park K, Oh MK, Kim GB, Kim HU, Lee SY, Park SJ, Joo JC. 2018. Metabolic engineering of Corynebacterium glutamicum for the production of glutaric acid, a C5 dicarboxylic acid platform chemical. Metab Eng 51:99–109. doi:10.1016/j.ymben.2018.08.007. - DOI - PubMed
-
- Vafaeezadeh M, Hashemi MM. 2016. A non-cyanide route for glutaric acid synthesis from oxidation of cyclopentene in the ionic liquid media. Process Saf Environ Prot 100:203–207. doi:10.1016/j.psep.2016.01.011. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
