Arabidopsis ALIX Regulates Stomatal Aperture and Turnover of Abscisic Acid Receptors
- PMID: 31363038
- PMCID: PMC6790096
- DOI: 10.1105/tpc.19.00399
Arabidopsis ALIX Regulates Stomatal Aperture and Turnover of Abscisic Acid Receptors
Abstract
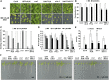
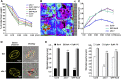
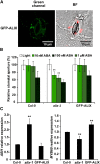
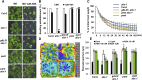
The plant endosomal trafficking pathway controls the abundance of membrane-associated soluble proteins, as shown for abscisic acid (ABA) receptors of the PYRABACTIN RESISTANCE1/PYR1-LIKE/REGULATORY COMPONENTS OF ABA RECEPTORS (PYR/PYL/RCAR) family. ABA receptor targeting for vacuolar degradation occurs through the late endosome route and depends on FYVE DOMAIN PROTEIN REQUIRED FOR ENDOSOMAL SORTING1 (FYVE1) and VACUOLAR PROTEIN SORTING23A (VPS23A), components of the ENDOSOMAL SORTING COMPLEX REQUIRED FOR TRANSPORT-I (ESCRT-I) complexes. FYVE1 and VPS23A interact with ALG-2 INTERACTING PROTEIN-X (ALIX), an ESCRT-III-associated protein, although the functional relevance of such interactions and their consequences in cargo sorting are unknown. In this study we show that Arabidopsis (Arabidopsis thaliana) ALIX directly binds to ABA receptors in late endosomes, promoting their degradation. Impaired ALIX function leads to altered endosomal localization and increased accumulation of ABA receptors. In line with this activity, partial loss-of-function alix-1 mutants display ABA hypersensitivity during growth and stomatal closure, unveiling a role for the ESCRT machinery in the control of water loss through stomata. ABA-hypersensitive responses are suppressed in alix-1 plants impaired in PYR/PYL/RCAR activity, in accordance with ALIX affecting ABA responses primarily by controlling ABA receptor stability. ALIX-1 mutant protein displays reduced interaction with VPS23A and ABA receptors, providing a molecular basis for ABA hypersensitivity in alix-1 mutants. Our findings unveil a negative feedback mechanism triggered by ABA that acts via ALIX to control the accumulation of specific PYR/PYL/RCAR receptors.
© 2019 American Society of Plant Biologists. All rights reserved.
Figures




Comment in
-
ALIX(ir) of Life: The Pivotal Role of ALIX in Regulating Plant Responses to Abscisic Acid.Plant Cell. 2019 Oct;31(10):2291-2292. doi: 10.1105/tpc.19.00629. Epub 2019 Aug 23. Plant Cell. 2019. PMID: 31444311 Free PMC article. No abstract available.
References
-
- Antoni R., Rodriguez L., Gonzalez-Guzman M., Pizzio G.A., Rodriguez P.L. (2011). News on ABA transport, protein degradation, and ABFs/WRKYs in ABA signaling. Curr. Opin. Plant Biol. 14: 547–553. - PubMed
-
- Antoni R., Gonzalez-Guzman M., Rodriguez L., Peirats-Llobet M., Pizzio G.A., Fernandez M.A., De Winne N., De Jaeger G., Dietrich D., Bennett M.J., Rodriguez P.L. (2013). PYRABACTIN RESISTANCE1-LIKE8 plays an important role for the regulation of abscisic acid signaling in root. Plant Physiol. 161: 931–941. - PMC - PubMed
-
- Babst M. (2005). A protein’s final ESCRT. Traffic 6: 2–9. - PubMed
-
- Babst M., Katzmann D.J., Estepa-Sabal E.J., Meerloo T., Emr S.D. (2002a). Escrt-III: An endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell 3: 271–282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

