Catalytic enantioselective addition of organometallics to unprotected carboxylic acids
- PMID: 31363092
- PMCID: PMC6667444
- DOI: 10.1038/s41467-019-11345-z
Catalytic enantioselective addition of organometallics to unprotected carboxylic acids
Abstract
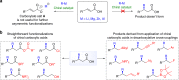
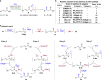
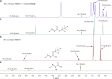
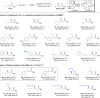
Conjugate addition of organometallics to carbonyl based Michael acceptors is a widely used method that allows the building of new carbon-carbon (C-C) bonds and the introduction of chirality in a single step. However, conjugate additions to the simplest Michael acceptors, namely unprotected, unsaturated carboxylic acids, are considered to be prohibited by the fact that acid-base reactions overpower any other type of reactivity, including nucleophilic addition. Here we describe a transient protecting group strategy that allows efficient catalytic asymmetric additions of organomagnesium reagents to unprotected α,β-unsaturated carboxylic acids. This unorthodox pathway is achieved by preventing the formation of unreactive carboxylate salts by means of a reactive intermediate, allowing modifications of the carbon chain to proceed unhindered, while the stereochemistry is controlled with a chiral copper catalyst. A wide variety of β-chiral carboxylic acids, obtained with excellent enantioselectivities and yields, can be further transformed into valuable molecules through for instance catalytic decarboxylative cross-coupling reactions.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Maag, H. Prodrugs of Carboxylic Acids (Springer, New York, 2007).
-
- Gminsights.com. Carboxylic Acid Market Size, Share - Industry Outlook Report 2024. https://www.gminsights.com/industry-analysis/carboxylic-acid-market [Accessed 26 June 2019] (2019).
-
- Perlmutter P. Conjugate Addition Reactions in Organic Synthesis. Pergamon, Oxford: Tetrahedron Organic Chemistry, Series 9; 1992.
-
- Alexakis, A., Krause, N. & Woodward, S. Copper-Catalyzed Asymmetric Synthesis (Wiley-VCH, Weinheim, Germany, 2014).
Publication types
LinkOut - more resources
Full Text Sources

