Standard screening methods underreport AAV-mediated transduction and gene editing
- PMID: 31363095
- PMCID: PMC6667494
- DOI: 10.1038/s41467-019-11321-7
Standard screening methods underreport AAV-mediated transduction and gene editing
Abstract
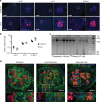
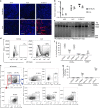
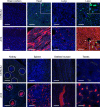
Conventional methods to discern adeno-associated virus (AAV) vector transduction patterns are based on high, stable expression of a reporter gene. As a consequence, conventionally described tropisms omit cell types that undergo transient transduction, or have low but undetectable levels of reporter expression. This creates a blind spot for AAV-based genome editing applications because only minimal transgene expression is required for activity. Here, we use editing-reporter mice to fill this void. Our approach sensitively captures both high and low transgene expression from AAV vectors. Using AAV8 and other serotypes, we demonstrate the superiority of the approach in a side-by-side comparison with traditional methods, demonstrate numerous, previously unknown sites of AAV targeting, and better predict the gene editing footprint after AAV-CRISPR delivery. We anticipate that this system, which captures the full spectrum of transduction patterns from AAV vectors in vivo, will be foundational to current and emerging AAV technologies.
Conflict of interest statement
B.L.D. is a founder of Spark Therapeutics and Talee Bio, Inc. and is on the advisory boards of Homology Medicines, Prevail Therapeutics, Sarepta Therapeutics, Intellia Therapeutics and Axovant Gene Therapies. The remaining authors declare no competing interests.
Figures



Similar articles
-
CRISPR-Mediated Integration of Large Gene Cassettes Using AAV Donor Vectors.Cell Rep. 2017 Jul 18;20(3):750-756. doi: 10.1016/j.celrep.2017.06.064. Cell Rep. 2017. PMID: 28723575 Free PMC article.
-
Use of AAV Vectors for CRISPR-Mediated In Vivo Genome Editing in the Retina.Methods Mol Biol. 2019;1950:123-139. doi: 10.1007/978-1-4939-9139-6_7. Methods Mol Biol. 2019. PMID: 30783971 Free PMC article.
-
Use of CRISPR/Cas9-mediated disruption of CNS cell type genes to profile transduction of AAV by neonatal intracerebroventricular delivery in mice.Gene Ther. 2021 Aug;28(7-8):456-468. doi: 10.1038/s41434-021-00223-3. Epub 2021 Feb 22. Gene Ther. 2021. PMID: 33612827 Free PMC article.
-
AAV Vectorization of DSB-mediated Gene Editing Technologies.Curr Gene Ther. 2016;16(3):207-19. doi: 10.2174/1566523216666160602213738. Curr Gene Ther. 2016. PMID: 27280971 Review.
-
CRISPR Systems Suitable for Single AAV Vector Delivery.Curr Gene Ther. 2022;22(1):1-14. doi: 10.2174/1566523221666211006120355. Curr Gene Ther. 2022. PMID: 34620062 Review.
Cited by
-
Preclinical evaluation of tissue-selective gene therapies for congenital generalised lipodystrophy.Gene Ther. 2024 Sep;31(9-10):445-454. doi: 10.1038/s41434-024-00471-z. Epub 2024 Jul 28. Gene Ther. 2024. PMID: 39069561 Free PMC article.
-
CRISPR targeting of mmu-miR-21a through a single adeno-associated virus vector prolongs survival of glioblastoma-bearing mice.Mol Ther. 2025 Jan 8;33(1):133-151. doi: 10.1016/j.ymthe.2024.11.023. Epub 2024 Nov 19. Mol Ther. 2025. PMID: 39563028
-
Long-term in vitro monitoring of AAV-transduction efficiencies in real-time with Hoechst 33342.PLoS One. 2024 Mar 1;19(3):e0298173. doi: 10.1371/journal.pone.0298173. eCollection 2024. PLoS One. 2024. PMID: 38427668 Free PMC article.
-
Drug delivery systems for CRISPR-based genome editors.Nat Rev Drug Discov. 2023 Nov;22(11):875-894. doi: 10.1038/s41573-023-00762-x. Epub 2023 Sep 18. Nat Rev Drug Discov. 2023. PMID: 37723222 Review.
-
Spatial genomics of AAV vectors reveals mechanism of transcriptional crosstalk that enables targeted delivery of large genetic cargo.Nat Biotechnol. 2025 Mar 20. doi: 10.1038/s41587-025-02565-4. Online ahead of print. Nat Biotechnol. 2025. PMID: 40113953
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

