Country-wide medical records infer increased allergy risk of gastric acid inhibition
- PMID: 31363098
- PMCID: PMC6667461
- DOI: 10.1038/s41467-019-10914-6
Country-wide medical records infer increased allergy risk of gastric acid inhibition
Abstract
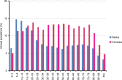
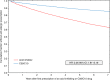
Gastric acid suppression promotes allergy in mechanistic animal experiments and observational human studies, but whether gastric acid inhibitors increase allergy incidence at a population level remains uncharacterized. Here we aim to assess the use of anti-allergic medication following prescription of gastric acid inhibitors. We analyze data from health insurance records covering 97% of Austrian population between 2009 and 2013 on prescriptions of gastric acid inhibitors, anti-allergic drugs, or other commonly prescribed (lipid-modifying and antihypertensive) drugs as controls. Here we show that rate ratios for anti-allergic following gastric acid-inhibiting drug prescriptions are 1.96 (95%CI:1.95-1.97) and 3.07 (95%-CI:2.89-3.27) in an overall and regional Austrian dataset. These findings are more prominent in women and occur for all assessed gastric acid-inhibiting substances. Rate ratios increase from 1.47 (95%CI:1.45-1.49) in subjects <20 years, to 5.20 (95%-CI:5.15-5.25) in > 60 year olds. We report an epidemiologic relationship between gastric acid-suppression and development of allergic symptoms.
Conflict of interest statement
The authors declare no competing interests. The funders played no role in the design of the study, in the collection, analysis or interpretation of data, or drafting or writing of the manuscript, in the decision to submit the paper for publication, and did not review or approve the manuscript before submission.
Figures

Comment in
-
Machen PPI jetzt auch noch allergisch?MMW Fortschr Med. 2019 Nov;161(19):34. doi: 10.1007/s15006-019-1055-x. MMW Fortschr Med. 2019. PMID: 31691225 Review. German. No abstract available.
-
Acid inhibitors and allergy: comorbidity, causation and confusion.Nat Commun. 2020 Aug 7;11(1):3950. doi: 10.1038/s41467-020-17831-z. Nat Commun. 2020. PMID: 32769978 Free PMC article. No abstract available.
-
Reply to "Acid inhibitors and allergy: comorbidity, causation and confusion".Nat Commun. 2020 Aug 7;11(1):3949. doi: 10.1038/s41467-020-17830-0. Nat Commun. 2020. PMID: 32770076 Free PMC article. No abstract available.
Similar articles
-
First evidence of a possible association between gastric acid suppression during pregnancy and childhood asthma: a population-based register study.Clin Exp Allergy. 2009 Feb;39(2):246-53. doi: 10.1111/j.1365-2222.2008.03125.x. Epub 2008 Dec 9. Clin Exp Allergy. 2009. PMID: 19134022
-
Gastric acid suppressants--too much of a good thing?Age Ageing. 2010 Jul;39(4):410-1. doi: 10.1093/ageing/afq057. Epub 2010 May 26. Age Ageing. 2010. PMID: 20507846 No abstract available.
-
Association Between Use of Acid-Suppressive Medications and Antibiotics During Infancy and Allergic Diseases in Early Childhood.JAMA Pediatr. 2018 Jun 4;172(6):e180315. doi: 10.1001/jamapediatrics.2018.0315. Epub 2018 Jun 4. JAMA Pediatr. 2018. PMID: 29610864 Free PMC article.
-
Gastric acidity inhibitors and the risk of intestinal infections.Curr Opin Gastroenterol. 2010 Jan;26(1):31-5. doi: 10.1097/MOG.0b013e328333d781. Curr Opin Gastroenterol. 2010. PMID: 19907324 Review.
-
Review article: potential gastrointestinal effects of long-term acid suppression with proton pump inhibitors.Aliment Pharmacol Ther. 2000 Jun;14(6):651-68. doi: 10.1046/j.1365-2036.2000.00768.x. Aliment Pharmacol Ther. 2000. PMID: 10848649 Review.
Cited by
-
Dysbiosis of the gut and lung microbiome has a role in asthma.Semin Immunopathol. 2020 Feb;42(1):75-93. doi: 10.1007/s00281-019-00775-y. Epub 2020 Feb 18. Semin Immunopathol. 2020. PMID: 32072252 Free PMC article. Review.
-
Optimizing drug selection from a prescription trajectory of one patient.NPJ Digit Med. 2021 Oct 20;4(1):150. doi: 10.1038/s41746-021-00522-4. NPJ Digit Med. 2021. PMID: 34671068 Free PMC article.
-
Acid-suppressive medication and incidence of chronic childhood immune-mediated diseases: A scoping review.Pediatr Allergy Immunol. 2023 Nov;34(11):e14042. doi: 10.1111/pai.14042. Pediatr Allergy Immunol. 2023. PMID: 38010007 Free PMC article.
-
Patients with Obesity and a History of Metformin Treatment Have Lower Influenza Mortality: A Retrospective Cohort Study.Pathogens. 2022 Feb 19;11(2):270. doi: 10.3390/pathogens11020270. Pathogens. 2022. PMID: 35215211 Free PMC article.
-
Repurposing anti-inflammasome NRTIs for improving insulin sensitivity and reducing type 2 diabetes development.Nat Commun. 2020 Sep 23;11(1):4737. doi: 10.1038/s41467-020-18528-z. Nat Commun. 2020. PMID: 32968070 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

