Genetic basis of functional variability in adhesion G protein-coupled receptors
- PMID: 31363148
- PMCID: PMC6667449
- DOI: 10.1038/s41598-019-46265-x
Genetic basis of functional variability in adhesion G protein-coupled receptors
Abstract
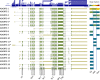
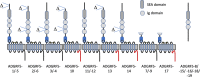
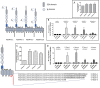
The enormous sizes of adhesion G protein-coupled receptors (aGPCRs) go along with complex genomic exon-intron architectures giving rise to multiple mRNA variants. There is a need for a comprehensive catalog of aGPCR variants for proper evaluation of the complex functions of aGPCRs found in structural, in vitro and animal model studies. We used an established bioinformatics pipeline to extract, quantify and visualize mRNA variants of aGPCRs from deeply sequenced transcriptomes. Data analysis showed that aGPCRs have multiple transcription start sites even within introns and that tissue-specific splicing is frequent. On average, 19 significantly expressed transcript variants are derived from a given aGPCR gene. The domain architecture of the N terminus encoded by transcript variants often differs and N termini without or with an incomplete seven-helix transmembrane anchor as well as separate seven-helix transmembrane domains are frequently derived from aGPCR genes. Experimental analyses of selected aGPCR transcript variants revealed marked functional differences. Our analysis has an impact on a rational design of aGPCR constructs for structural analyses and gene-deficient mouse lines and provides new support for independent functions of both, the large N terminus and the transmembrane domain of aGPCRs.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

