Circuit-based interventions in the dentate gyrus rescue epilepsy-associated cognitive dysfunction
- PMID: 31363737
- PMCID: PMC6736326
- DOI: 10.1093/brain/awz209
Circuit-based interventions in the dentate gyrus rescue epilepsy-associated cognitive dysfunction
Abstract
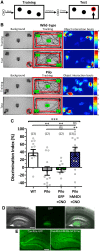
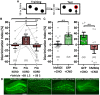
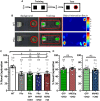
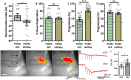
Temporal lobe epilepsy is associated with significant structural pathology in the hippocampus. In the dentate gyrus, the summative effect of these pathologies is massive hyperexcitability in the granule cells, generating both increased seizure susceptibility and cognitive deficits. To date, therapeutic approaches have failed to improve the cognitive symptoms in fully developed, chronic epilepsy. As the dentate's principal signalling population, the granule cells' aggregate excitability has the potential to provide a mechanistically-independent downstream target. We examined whether normalizing epilepsy-associated granule cell hyperexcitability-without correcting the underlying structural circuit disruptions-would constitute an effective therapeutic approach for cognitive dysfunction. In the systemic pilocarpine mouse model of temporal lobe epilepsy, the epileptic dentate gyrus excessively recruits granule cells in behavioural contexts, not just during seizure events, and these mice fail to perform on a dentate-mediated spatial discrimination task. Acutely reducing dorsal granule cell hyperactivity in chronically epileptic mice via either of two distinct inhibitory chemogenetic receptors rescued behavioural performance such that they responded comparably to wild type mice. Furthermore, recreating granule cell hyperexcitability in control mice via excitatory chemogenetic receptors, without altering normal circuit anatomy, recapitulated spatial memory deficits observed in epileptic mice. However, making the granule cells overly quiescent in both epileptic and control mice again disrupted behavioural performance. These bidirectional manipulations reveal that there is a permissive excitability window for granule cells that is necessary to support successful behavioural performance. Chemogenetic effects were specific to the targeted dorsal hippocampus, as hippocampal-independent and ventral hippocampal-dependent behaviours remained unaffected. Fos expression demonstrated that chemogenetics can modulate granule cell recruitment via behaviourally relevant inputs. Rather than driving cell activity deterministically or spontaneously, chemogenetic intervention merely modulates the behaviourally permissive activity window in which the circuit operates. We conclude that restoring appropriate principal cell tuning via circuit-based therapies, irrespective of the mechanisms generating the disease-related hyperactivity, is a promising translational approach.
Keywords: chemogenetics; circuit-based therapy; cognitive deficits; dentate gyrus; temporal lobe epilepsy.
© The Author(s) (2019). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures




References
-
- Bender RA, Soleymani S V, Brewster AL, Nguyen ST, Beck H, Mathern GW et al. Enhanced expression of a specific hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN) in surviving dentate gyrus granule cells of human and experimental epileptic hippocampus. J Neurosci 2003; 23: 6826–36. - PMC - PubMed
-
- Bertram EH. Functional anatomy of spontaneous seizures in a rat model of limbic epilepsy. Epilepsia 1997; 38: 95–105. - PubMed
-
- Blumcke I, Suter B, Behle K, Kuhn R, Schramm J, Elger CE et al. Loss of Hilar Mossy cells in ammon’s horn sclerosis. Epilepsia 2000; 41: S174–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

