Expanding Hepatitis C Virus Care and Cure: National Experience Using a Clinical Pharmacist-Driven Model
- PMID: 31363775
- PMCID: PMC6667715
- DOI: 10.1093/ofid/ofz316
Expanding Hepatitis C Virus Care and Cure: National Experience Using a Clinical Pharmacist-Driven Model
Abstract
Background: The US National Viral Hepatitis Action Plan depends on additional providers to expand hepatitis C virus (HCV) treatment capacity in order to achieve elimination goals. Clinical pharmacists manage treatment and medication within interdisciplinary teams. The study's objective was to determine sustained virologic response (SVR) rates for clinical pharmacist-delivered HCV therapy in an open medical system.
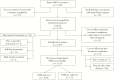
Methods: Investigators conducted a multicenter retrospective cohort study of patients initiating direct-acting antivirals from January 1, 2014, through March 12, 2018. Data included demographics, comorbidities, treatment, and clinical outcomes. The primary outcome of SVR was determined for patients initiating (intent-to-treat) and those who completed (per-protocol) treatment. Chi-square tests were conducted to identify associations between SVR and adverse reactions, drug-drug interactions, and adherence.
Results: A total of 1253 patients initiated treatment; 95 were lost to follow-up, and 24 discontinued therapy. SVR rates were 95.1% (1079/1134) per protocol and 86.1% (1079/1253) intent to treat. The mean age (SD) was 57.4 (10.1) years, the mean body mass index (SD) was 28.7 (6.2) kg/m2, 63.9% were male, 53.7% were black, 40.3% were cirrhotic, 88.4% were genotype 1, and 81.6% were treatment-naïve. Patients missing ≥1 dose had an SVR of 74.9%; full adherence yielded 90% (P < .0001).
Conclusions: HCV treatment by clinical pharmacists in an open medical system resulted in high SVR rates comparable to real-world studies with specialists and nonspecialists. These findings demonstrate the success of a clinical pharmacist-delivered method for HCV treatment expansion and elimination.
Keywords: HCV elimination; clinical pharmacists; direct-acting antiviral; hepatitis C virus; interdisciplinary.
© The Author(s) 2019. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures

References
-
- Centers for Disease Control and Prevention. Surveillance for viral hepatitis – United States, 2016. Available at: https://www.cdc.gov/hepatitis/statistics/2016surveillance/index.htm. Accessed 20 June 2019.
-
- Ottman AA, Townsend ML, Hashem MG, et al. Incidence of drug interactions identified by clinical pharmacists in veterans initiating treatment for chronic hepatitis C infection. Ann Pharmacother 2018; 52:763–8. - PubMed
LinkOut - more resources
Full Text Sources

