The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms
- PMID: 31363792
- PMCID: PMC6813288
- DOI: 10.1007/s00011-019-01273-5
The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms
Abstract
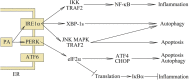
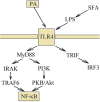
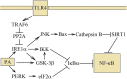
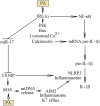
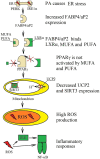
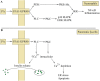
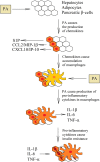
Palmitic acid is a saturated fatty acid whose blood concentration is elevated in obese patients. This causes inflammatory responses, where toll-like receptors (TLR), TLR2 and TLR4, play an important role. Nevertheless, palmitic acid is not only a TLR agonist. In the cell, this fatty acid is converted into phospholipids, diacylglycerol and ceramides. They trigger the activation of various signaling pathways that are common for LPS-mediated TLR4 activation. In particular, metabolic products of palmitic acid affect the activation of various PKCs, ER stress and cause an increase in ROS generation. Thanks to this, palmitic acid also strengthens the TLR4-induced signaling. In this review, we discuss the mechanisms of inflammatory response induced by palmitic acid. In particular, we focus on describing its effect on ER stress and IRE1α, and the mechanisms of NF-κB activation. We also present the mechanisms of inflammasome NLRP3 activation and the effect of palmitic acid on enhanced inflammatory response by increasing the expression of FABP4/aP2. Finally, we focus on the consequences of inflammatory responses, in particular, the effect of TNF-α, IL-1β and IL-6 on insulin resistance. Due to the high importance of macrophages and the production of proinflammatory cytokines by them, this work mainly focuses on these cells.
Keywords: Inflammation; Insulin resistance; Macrophage; Obesity; Palmitic acid; Saturated fatty acid.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Mook S, Halkes CJ, Bilecen S, Cabezas MC. In vivo regulation of plasma free fatty acids in insulin resistance. Metabolism. 2004;53:1197–2001. - PubMed
-
- Pankow JS, Duncan BB, Schmidt MI, Ballantyne CM, Couper DJ, Hoogeveen RC, Golden SH, Atherosclerosis Risk in Communities Study Fasting plasma free fatty acids and risk of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2004;27:77–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

